Fig. 31.1
Proposed staging system for the menopausal transition (Reprinted from fertility and sterility, 76/5, Soules et al. [26], Copyright 2001, with permission from Elsevier)
Mitchell and colleagues modified their framework following the Staging Reproductive Aging Workshop (STRAW) (see Soules et al. [26] for details) and in collaboration with the ReSTAGE investigators. Figure 31.2 includes a menstrual calendar from a woman in the late reproductive stage (STRAW -3), showing regularity of her menstrual cycles over a year. Figure 31.3 includes data from a woman who is experiencing irregularity of 7 days or more between consecutive menstrual cycles, indicating that she is now in the early menopausal transition stage. Figure 31.4 illustrates cycles for a woman who has begun skipping periods with at least 60 days of amenorrhea between periods, the criterion for late menopausal transition stage. Later the ReSTAGE project investigators analyzed data from the Melbourne Women’s Health Project, the Study of Women and Health Across the Nation (SWAN), the Tremin Trust Data, and the Seattle Midlife Women’s Health Study. They validated that a break in cyclicity manifested by a difference of 7 or more days in cycle length between sequential cycles that reoccurred within the next 10 bleeding segments was a good indicator for the beginning of the early phase of the menopausal transition. Moreover, their work indicated that a period of 60 or more days of amenorrhea that reoccurred within the next 10 bleeding segments for women less than 45 years of age signaled the entry into the late stage of the menopausal transition [27–30].
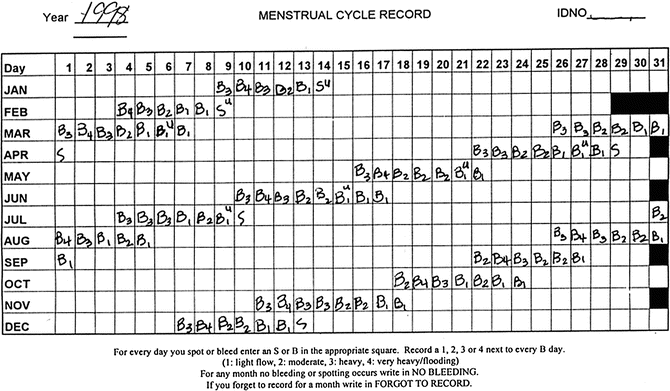
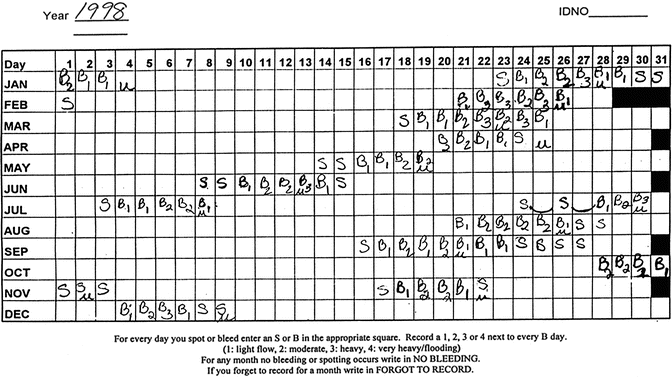
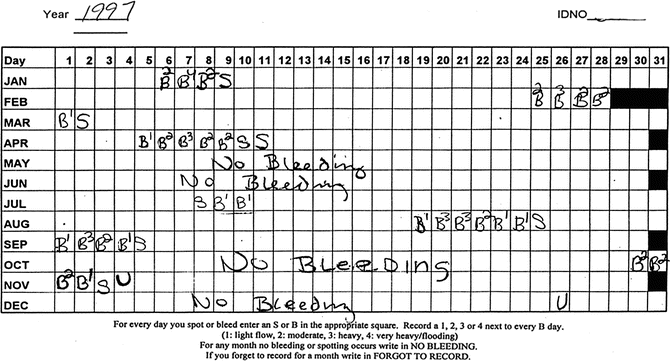

Fig. 31.2
Menstrual calendar, late reproductive stage

Fig. 31.3
Menstrual calendar, early menopausal transition stage

Fig. 31.4
Menstrual calendar, late menopausal transition stage
Women do not experience the final menstrual period at the same age, nor do they experience the stages of reproductive aging at specific ages. Table 31.1 includes the ages of onset and duration of the stages of the menopausal transition based on the Seattle Midlife Women’s Health Study. Of interest is that the early stage required an average of 2.9 years and the late stage an average of 2.5 years. When the early and late menopausal transition stages for women in the SMWHS were added together, the average duration of the menopausal transition (early + late) was 5.3 years, (SD = 1.8 years with a range from 1.1 to 9.8 years) (Mitchell and Woods, unpublished data). Similar data from other longitudinal cohorts will support the universality or variability in these data across populations.
Table 31.1
Age of onset and duration of menopausal transition stages: observations from the Seattle Midlife Women’s Health Study (2011)
Early menopausal transition stage | Late menopausal transition stage | Early postmenopause | |
|---|---|---|---|
Age of onset | N = 125 | N = 131 | N = 112 |
M,SD | 46.3, 3.6 | 49.5, 2.8 | 52.1, 2.9 |
Median | 46.8 | 49.8 | 52.4 |
Duration | N = 83 | N = 82 | – |
M, SD, | 2.9, 1.5 | 2.5, 1.3 | |
Median | 2.6 | 2.3 | |
Range | 0.2–6.5 | 0.4–7.0 |
31.4 Endocrine Changes During the Menopausal Transition and Early Postmenopause
Staging reproductive aging, anchored by changes observed in menstrual cycle regularity, provided a framework within which to examine changes occurring in the ovarian (estrogens, progesterone, testosterone, Mullerian-inhibiting hormone (MIH), and inhibins) and pituitary hormones (FSH and LH). Santoro’s early and careful description of endocrine levels across the menstrual cycles of women approaching menopause and those who had experienced their final menstrual period stimulated awareness of the changes in endocrine levels, including the realization that some women experienced episodes of hyper-estrogen levels before their final period [16].
SWAN investigators found there was a period of approximately four years during which the maximal changes occur at the greatest rate in both FSH and estradiol, with the changes in FSH preceding the changes in estradiol. After this 4-year period of maximum changes, both FSH and E2 levels stabilize [31, 32]. Acceleration of the FSH rise occurs 2 years before the FMP with deceleration beginning immediately before the FMP and stabilizing 2 years after the FMP [32], consistent with earlier findings of Sowers and colleagues [33]. Estradiol concentrations did not change substantially until about 2 years before the FMP when it began decreasing with the maximal rate of change occurring at the FMP and then decelerating to achieve stability about 2 years after FMP. Obesity, smoking behavior, and being Chinese or Japanese were associated with variation in estradiol levels, but not with the pattern of estradiol change. In obese women, initial acceleration of FSH occurred slightly later (at 5.45 years before FMP) and was attenuated compared to that observed in nonobese women.
In the daily hormone sub-study of SWAN, Santoro found that cycles become anovulatory prior to the FMP with progesterone levels dropping as women ovulated more irregularly or ceased ovulation [34]. Although the precise cause of menstrual cessation is not yet clear, we know that estradiol levels drop as ovulation decreases in frequency and FSH rises in response to ovarian signals from inhibins and Anti-Müllerian Hormone [32–36]. AMH is produced in growing ovarian follicles, a direct indicator of ovarian reserve, and becomes nondetectable 5 years prior to the final menstrual period. Inhibin B is produced by small antral follicles, indicating growth of the antral follicle cohort. Inhibin B suppresses FSH secretion through negative feedback to the pituitary and declines to undetectable levels 4–5 years prior to the final menstrual period [37]. Work is still underway to determine which, if any, of these indicators is the best marker for predicting menopause.
Androgens, including testosterone and DHEAS, have also been studied in the SWAN population, revealing a rise in DHEAS during the late menopausal transition stage, followed by a decline during the early postmenopause [38, 39], Approximately 85 % of women had an increase in DHEAS between pre/early menopausal transition and late menopausal transition/early postmenopause [39]. DHEAS provides an important source of estrogen for women during the postmenopause as it is converted to estrone. Testosterone levels were relatively stable in the Melbourne Midlife Women’s Health cohort and the SWAN cohort [32, 38, 40].
Cortisol has also been shown to rise between the early menopausal transition stage and late menopausal transition stage in one study [41], but such a rise was not seen in the SWAN study [38]. Effects of both estrogens and androgens on women’s health after menopause have been explored recently and are discussed later in this chapter.
Lasley et al. [42] investigated the impact of adrenal contributions to sex steroids in the SWAN participants, finding that DHEAS, DHEA, and androstenediol increase at their greatest rate and are at peak variability during the years immediately before menopause when estradiol levels are low. Androstenediol is a prohormone for peripheral conversion to bioactive steroids, acting as a signal transducer in estrogen and androgen receptors. Adiol levels increase fivefold at the same time circulating estradiol levels are decreasing. Lasley proposed that disappearance of inhibin B rising FSH triggers an increase in delta-5 (androstenediol) production, producing a transition in metabolism from estrogenic to androgenic. SWAN participants exhibited high levels of androstenediol, about 100 time the levels of E2. Lasley proposed that the higher levels of Adiol, which has lower estrogenic bioactivity than estradiol, may be needed to compensate for lower estradiol levels during this period. Although the clinical significance of this increase in Adiol is not known at this time, the higher concentration of Adiol in the presence of lower estradiol levels in the years prior to the FMP could contribute to total circulating estrogen ligand pool in women during the MT.
Adrenal androgen dynamics may be key to understanding development of the metabolic syndrome. The relationship of both ovarian and adrenal steroids to health during the menopausal transition and postmenopause is not fully understood, and to date most researchers have studied changes in individual hormones and the ratio of estradiol to testosterone. As discussed later, it is may be the ratio of testosterone to estradiol that is the most important predictor of changes in cardiovascular disease risk. In addition, the promise of more sensitive assays for androgens and a more full understanding of the role of adrenal androgens as precursors of estrogens will enhance our concepts of endocrine changes during the menopausal transition on many health outcomes during the postmenopause.
31.5 Symptoms During the Menopausal Transition and Early Postmenopause
31.5.1 Hot Flashes
Women experience a variety of symptoms during the menopausal transition, including hot flashes and night sweats, often referred to as vasomotor symptoms. A hot flash is a sensation of intense heat, often accompanied by sweating and flushing. Hot flashes occur in an effort to dissipate heat characterized by vascular dilation. Voda [19] characterized the menopausal hot flash from women’s own experiences, exploring the question “who is the woman who has hot flashes and what are the characteristics of the hot flashes?” Using data from daily self-reports, she sought to describe the frequency, duration, trigger, origin, spread, intensity, and method of coping with hot flashes. She found that no single pattern characterized women’s experiences. Although the majority of hot flashes began on the upper body, for example, the chest or face, some women noted their hot flashes started in other parts of their body. Hot flashes tended to spread to other areas on the upper body but for some women spread to the legs and arms or back. On average, a hot flash lasted about 3 min. Women distinguished between mild, moderate, and severe hot flashes. Their coping strategies related to the duration and severity of their hot flashes. Internal strategies included ingesting a cold beverage. External strategies involved fanning oneself, showering, or opening a window. Some hot flash triggers included sleeping, work activities, recreation and relaxation, and housework. Voda’s study emphasized the variability in women’s hot flash experiences and how they managed them. In collaboration with Kay and others, Voda extended this work to characterize the experiences of both Mexican American and Anglo women [43]. They found that although Anglo women experienced hot flashes negatively, Mexican American women found positive components of meaning, for example, they viewed menopause as a natural part of life, indicating they could no longer have children, and meaning they could be confident they would not become pregnant again [43].
Hot flashes that women reported in laboratory studies were associated with a rise in skin temperature and skin conductance levels, elevated heart rate and respiratory rate, and reduced blood pH. Laboratory studies also revealed autonomic nervous system activation, such as occurs with a stress response, in mediating vasodilation and elaboration of norepinephrine following the hot flash [44–46]. Potent vasodilators such as calcitonin gene-related peptide are released during hot flashes, but not during exercise or sweating [47]. Although the etiology of hot flashes and the mechanisms stimulating vasodilation remain unclear, most investigators suggest that estrogen withdrawal or changing estrogen levels is involved.
The effects of changing estrogen levels on blood vessel structure and function are beginning to be characterized by SWAN investigators focusing on the relationship of the menopausal transition and associated physiological changes to heart disease risk. Thurston et al. [48] found that women who had hot flashes experience lower heart rate variability during the flash, indicating that the parasympathetic nervous system, which helps influence return to normal heart rate after a stressful experience, may function differently in women with hot flashes than in women who do not have hot flashes. Woods and colleagues found that women experiencing a cluster of symptoms with severe hot flashes had higher norepinephrine levels than those experiencing low severity symptoms [49]. Thurston also found that women who have hot flashes have less expansion of their arteries when blood flow is increased than do women without hot flashes [47]. Hot flashes also have been linked to calcification of the aorta seen in heart disease [48, 50] and increased carotid intima-medial thickness among midlife women [51]. There is also an association with adiposity and hot flashes that suggests that women with greater adiposity experience more severe hot flashes [52–55]. In contrast, when hot flash monitors are used to measure sternal skin conductance, higher BMI and waist circumference were associated with fewer hot flashes, but only among older women in the study [52]. In addition, there is evidence that women who experienced hot flashes have higher levels of tPA and factor VII than those without hot flashes [56]. Taken together, this work is beginning to suggest the role of hot flashes as a marker for subclinical heart disease.
Hot flashes are associated with both increased FSH and lower estrogen levels and also are associated with increased bone turnover during the menopausal transition. During the early and late stages of the menopausal transition, women with the most frequent hot flashes tended to have higher N-telopeptide levels, a marker of bone loss [57]. Moreover, low estrogen levels as well as higher FSH levels have been associated with higher levels of interleukins, for example, IL1B, linking both gonadotropins and estrogen to immune response [58] as well as to hot flashes.
As Voda found, hot flashes may be barely noticeable or severe, resulting in a high degree of variability of the experience among women [19, 59]. For some, hot flashes are associated with impaired quality of life, disturbed sleep, irritability, and depressed mood, among other symptoms [60]. Thurston found that women who were most bothered by hot flashes were those with more negative affect, greater symptom sensitivity, sleep problems, poorer health, duration of hot flashes, younger age, and African American race. Bother associated with night sweats was associated with sleep problems and night sweats duration [60]. For many women, hot flashes are sufficiently bothersome to lead them to seek health care during the menopausal transition [61, 62]. Although the majority of women in most population-based studies experience hot flashes [63], it is unclear how long they persist. Barnabei and colleagues [64] found that between 23 and 37 % of participants in the Women’s Health Initiative Study who were in their 60s and 11–20 % of women in their 70s reported hot flashes. Some participants in the Melbourne study reported hot flashes for as long as 10 years after the FMP, although the average duration was 5 years [65].
In the Seattle Midlife Women’s Health Study, hot flash severity increased as women entered the late menopausal transition stage or early postmenopause. Those who used hormone therapy, had a longer duration of the early menopausal transition stage, were older at the time of their final menstrual period, and had higher levels of FSH had more severe hot flashes. Anxiety was also associated with hot flash severity. Older age at entry into the early menopausal transition stage and higher urinary estrogen (estrone) levels were associated with decreased hot flash severity.
Psychosocial/mood (stress, depressed mood) and lifestyle variables (BMI, activity level, sleep, alcohol use) were not associated with hot flash severity in this study [59]. In contrast, in daily diary studies, women reported negative affect on the same day and the day after they reported hot flashes, suggesting that negative cognitive appraisal of hot flashes and perhaps other associated symptoms are linked to subsequent experiences of negative affect [66]. Other investigations have revealed that body mass index (BMI) [67], anxiety [68, 69], and other lifestyle behaviors have been associated with hot flash severity. As one example, women who smoked reported more severe hot flashes [67].
31.5.2 Sleep Symptoms
In addition to hot flashes, women commonly experience sleep symptoms during the menopausal transition and early postmenopause. Shaver was the first to study sleep during the perimenopause using polysomnographic methods in a sleep laboratory, discovering the relationship between sleep problems and ongoing stressful life events and anxiety [70–73]. In the Seattle Midlife Women’s Health Study, women who experienced more severe difficulty going to sleep had several other symptoms such as anxiety, reported more stress, had a history of sexual abuse, rated their own health more poorly, and had greater caffeine intake and less alcohol use than women who did not have this problem. Neither estrogen nor FSH was related to difficulty getting to sleep. On the other hand, women who had more severe awakening during the night were older, were more likely to be in the late MT stage and early postmenopause, had higher FSH and lower estrogen (estrone) levels, and reported more severe hot flashes, depressed mood, anxiety, joint pain, backache, perceived stress, poorer overall health, less alcohol use, and a history of sexual abuse. Women who had more severe problems with awakening early (and not getting back to sleep) were older, reported more severe hot flashes, depressed mood, anxiety, joint pain, backache, and perceived stress, rated their health more poorly, and had higher epinephrine and lower urinary estrogen levels [74]. These findings are consistent with those of other contemporary studies [75–77]. Moreover, in the Seattle Midlife Women’s Health Study, sleep symptoms were related to other symptoms, including hot flashes, depressed mood, anxiety, and pain [74, 78]. Recent findings from a study of relationships between menopausal transition-related symptoms and EEG sleep measures indicate that hot flashes were associated with longer sleep time. Women with higher anxiety symptoms had longer sleep latency and lower sleep efficiency only if they also had hot flashes. Hot flashes and mood symptoms were unrelated to either delta sleep ratio or REM latency [79]. In this same study, elevated beta EEG power in the NREM and REM sleep in women during late perimenopause (late MT) and early postmenopause exceeded levels in pre (late reproductive stage) and early perimenopause (early MT). Elevated beta EEG power indicates increased arousal and disturbed sleep quality during the late perimenopause (late MT) and early postmenopause [80]. Sleep symptoms during the MT may be amenable to symptom management strategies that take into account women’s experiences of arousal and their ability to regulate it as well as efforts to promote women’s general health rather than focusing only on the MT.
31.5.3 Depressed Mood
Depressed mood is also a commonly experienced symptom during the menopausal transition and early postmenopause. In the Seattle Midlife Women’s Health Study, age was associated with slightly lower depressed mood (CES-D) scores. Being in the late MT stage was significantly related to experiencing depressed mood, although there was no effect of being in the early MT stage or the early postmenopause. Hot flash severity, life stress, family history of depression, history of postpartum blues, sexual abuse history, BMI, and use of antidepressants were also individually related to depressed mood. Neither FSH nor estrogen was related to depressed mood [81–83]. Several investigator groups have now identified the menopausal transition as a period of vulnerability to depressed mood [84–87]. There is a suggestion that variability of hormonal levels (estrogen, FSH) is related to depressed mood, although there is no evidence that estrogen levels themselves are related [87–89]. Of interest is that testosterone rise has been associated with depressed mood in a subset of the SWAN study participants [85] and DHEAS associated with depressed mood symptoms but not major depression in the Penn Ovarian Aging Study participants [90]. A recent assessment of SWAN participants who responded to a Structured Clinical Interview for DSM-IV Axis Disorders (SCID) revealed that compared to women who were premenopausal, those who were in the perimenopause or early postmenopause were 2–4 times more likely to experience a major depressive episode, even when prior depression history, upsetting life events, psychotropic medication use, hot flashes, and serum levels or changes in reproductive hormone levels were taken into account. Although women in the late menopausal transition stage are vulnerable to depressed mood, factors that account for depressed mood earlier in the lifespan continue to have an important influence and should be considered in studies of etiology and therapeutics. Of interest is that depressive symptoms during midlife are related to progression of coronary artery calcification, a risk factor for cardiovascular disease [91].
31.5.4 Cognitive Symptoms
Women complain of forgetfulness and difficulty concentrating commonly during this period of life. Seattle Midlife Women’s Health Study participants who experienced more severe difficulty concentrating were slightly older, reported more anxiety, depressed mood, nighttime awakening, perceived stress, poorer perceived health, and were employed. The best predictors of forgetfulness included slightly older age, hot flashes, anxiety, depressed mood, awakening during the night, perceived stress, poorer perceived health, and history of sexual abuse.
Menopausal transition-related factors were not significantly associated with difficulty concentrating or forgetfulness. Considering women’s ages and the context in which they experience the menopausal transition may be helpful in understanding women’s experiences of cognitive symptoms [92]. Studies of functional changes in memory during the menopausal transition indicate that aside from a period of slightly reduced learning, there are no significant changes in memory function during this period of the lifespan. The minor slowing of learning during the late menopausal transition stage disappears during the early postmenopause [93–95].
31.5.5 Pain Symptoms
Although women experience pain symptoms throughout the lifespan, there is some evidence that pain symptoms are influenced by estrogen [96]. In the Seattle Midlife Women’s Health Study, women experienced a slight increase in back pain with age and a significant increase in back pain during the early and late MT stages and early postmenopause, but estrogen, FSH, and testosterone levels were unrelated to back pain [78]. Perceived stress and lower overnight urinary cortisol levels were associated with more severe back pain; history of sexual abuse and catecholamines did not have a significant effect. Those most troubled by symptoms of hot flashes, depressed mood, anxiety, nighttime awakening, and difficulty concentrating reported significantly greater back pain. Of the health-related factors, having worse perceived health, exercising more, using analgesics, and having a higher BMI were associated with more back pain, but alcohol use and smoking did not have significant effects. Having more formal education was associated with less back pain, but parenting, having a partner, and employment were unrelated. Age was associated with increased severity of joint pain, but menopausal transition-related factors, such as stage or hormone levels, were unrelated. Symptoms of hot flashes, nighttime awakening, depressed mood, and difficulty concentrating were each significantly associated with joint pain, as was poorer perceived health, more exercise, higher BMI, and greater analgesic use. History of sexual abuse was the only stress-related factor significantly related to joint pain severity. Based on these findings which are consistent with those of others [97–100], clinicians working with women traversing the menopausal transition should be aware that managing back and joint pain symptoms among midlife women requires consideration of their changing biology as well as their ongoing life challenges and health-related behaviors. Moreover, the relationship between pain and sleep symptoms should be considered, including sleep hygiene interventions [78].
31.5.6 Sexual Desire
There is a great deal of interest in the effects of the menopausal transition on sexual desire. In the Seattle Midlife Women’s Health Study, women experienced a significant decrease in sexual desire during the late MT stage and early PM [100]. Those with higher urinary estrone (E1G) and testosterone (T) reported significantly higher levels of sexual desire, whereas those with higher FSH levels reported significantly lower sexual desire. Women using hormone therapy also reported higher sexual desire. Those reporting higher perceived stress reported lower sexual desire, but history of sexual abuse did not have a significant effect. Those most troubled by symptoms of hot flashes, fatigue, depressed mood, anxiety, difficulty getting to sleep, early morning awakening, and awakening during the night also reported significantly lower sexual desire, but there was no effect of vaginal dryness. Women with better perceived health reported higher sexual desire, and those reporting more exercise and more alcohol intake also reported greater sexual desire. Having a partner was associated with lower sexual desire. Women’s sexual desire during the menopausal transition and early postmenopause is related to both her biology as well as the social situation in which she finds herself [101–103].
31.6 Interference with Daily Living and Symptoms
In addition to rating symptoms as severe or bothersome, many women indicate their symptoms interfere with many aspects of their daily lives, for example, work and relationships with family and friends [104]. In an effort to determine which sets of symptoms were most challenging for women, participants in the Seattle Midlife Women’s Health Study rated the degree to which how they felt each day interfered with their ability to work and their relationships. Hot flashes, depressed mood, anxiety, sleep problems, cognitive symptoms, and pain symptoms each contributed to interference, but the most influential factors interfering with work were stress levels and difficulty concentrating. The most influential factors interfering with relationships were stress, depressed mood, and problems concentrating [105].
31.6.1 Clusters of Symptoms During the Menopausal Transition
As studies of the menopausal transition and symptoms have progressed, it is increasingly evident that women experience multiple symptoms with some experiencing multiple severe symptoms. Moreover, researchers studying symptom have identified the importance of studying co-occurring symptoms or symptom clusters as a basis for identifying mechanisms that may be common to several symptoms or explain relationships among symptoms. As an example, Joffe and colleagues have found that induced hot flashes objectively recorded influence sleep efficiency, creating fragmentation. Subjective hot flashes are associated with perceived poor sleep quality [106]. In addition, investigators studying symptom clusters are concerned about identifying therapeutics that will maximize effects of an intervention on all or most symptoms and minimize the likelihood that a therapy will have positive effects on one symptom but exacerbate others [107]. Cray and colleagues identified three clusters of symptoms women experienced during the menopausal transition and early postmenopause [108]. Among these were clusters of (1) low severity symptoms of all types (hot flashes, mood, sleep disruption, cognitive, pain, and tension symptoms; (2) moderately severe hot flashes along with moderate levels of other symptoms; and (3) low severity hot flashes with moderate levels of all other symptoms (Fig. 31.5). The high hot flash cluster versus the low symptom severity cluster was associated with being in the late menopausal transition stage as well as with higher levels of FSH, lower levels of estrogen, higher norepinephrine, and lower epinephrine levels. The moderate severity symptom cluster versus the low severity cluster was associated only with having lower epinephrine levels [109].
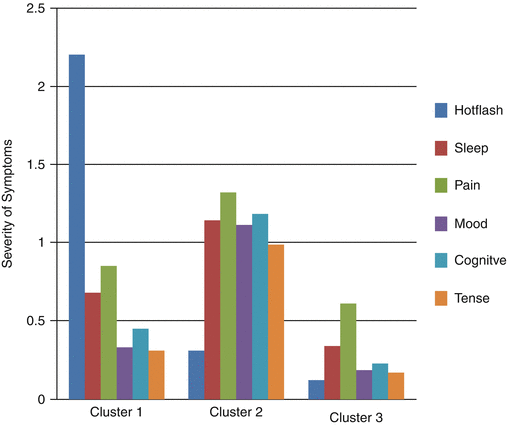

Fig. 31.5
Severity of Symptoms by Symptom Clusters Identified in the Seattle Midlife Women’s Health Study
In contrast to a symptom cluster, a syndrome is a pattern of symptoms that is presumably disease-specific and results from a common underlying mechanism. Avis and colleague demonstrated a lack of evidence for a “menopausal syndrome” despite some evidence linking some of the symptoms in clusters to endocrine dynamics during the menopausal transition [110].
Given the global nature of health care, it is important to focus on women’s experiences of menopause in many parts of the world. To date much of this body of work has been conducted in economically developed countries, often incorporating measures common to Western cultures. A detailed review of the symptoms women experience around the globe is beyond the scope of this chapter. Nonetheless, Leidy Sievert [111] has led development of research culminating in identification of ways in which diverse populations of women experience menopause. Her work includes a biocultural model in which environment, culture, and biology intersect to influence the expression of hot flashes. Leidy Sievert elaborates that environment prompts consideration of the climate and altitude in which women live their lives and that culture warrants consideration of practices related to marriage, religion, attitudes, medicalization, hysterectomy practices, smoking, reproductive patterns, and diet. Finally, because different populations of the world have different genetic characteristics, they also may have differing hormone levels and sweating patterns. Thus, the variation across populations and the variation within populations of women are complex and together influence women’s individual experiences. Indeed, Leidy Sievert points out that cross-country comparative studies illustrate the differences between cultures, while cross-cultural study of menopause can facilitate understanding of women’s place in society and the influence of social context on symptom experience [112]. In a comparison of symptom experiences across countries, women from different countries report some similar symptoms but may cluster their symptoms differently. For example, expression of somatic with emotional complaints varies across populations, possibly reflecting comfort with expression of emotional symptoms [113]. Both Avis [110] and Locke [114] have provided compelling data about the diversity of the menopause experience and associated symptoms.
31.7 Stress and the Menopausal Transition
Given the nature of symptoms that women experience during the menopausal transition, one might ask whether the menopausal transition, itself, is stressful. We found that there was little change in perceived stress during the early and late menopausal transition stages. Instead, as women aged, those who were employed and had a history of sexual abuse and depressed mood experienced greater stress. Those who experienced an improvement in the burdens associated with their roles, more social support and more adequate incomes reported less stress. Those who appraised aging changes in their bodies as negative and perceived their health as poorer had higher stress levels [115]. These findings are consistent with findings of other studies of women that implicate exposure to stress to symptom experience during this part of the lifespan. Clinicians working with women traversing the menopausal transition should remain vigilant to the social circumstances of women’s lives, as well as focusing on the social and endocrine features of this transition. Of interest is that when we asked women who had participated in the Seattle Midlife Women’s Health Study for 15 years what was the most challenging aspect of their lives during that period, only one said that it was the menopausal transition (Woods and Mitchell, unpublished).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





