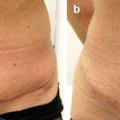
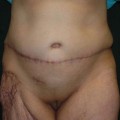
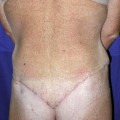
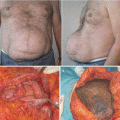
In light of this formula, patients were grouped as group A, perimenstrual (0–7, 21–28. days); group B, periovulatory (8–20. days); and group C, postmenopausal. The main focus was on premenopausal women to determine a relationship between perioperative blood loss and the menstrual phase. Postmenopausal patients were also added for comparison. Patients’ age, body mass index (BMI), and weight of the resected abdominal tissue were also considered as other factors that could influence the amount of bleeding and were therefore also evaluated statistically.
36.3 Results
Groups A and B were both comprised of 15 patients, while group C had 11 patients. The average age was 44 ± 7 years (range 32–61 years). Average BMI was calculated as 28 ± 4 kg/m2 (22–35 kg/m2), while the average weight of resected abdominal tissue was 2009 ± 798 g (880–3950 g). There was no significant difference among groups regarding BMI values and the weight of the resected abdominal tissue (p > 0.05).
The average intraoperative blood loss and postoperative 48-h total blood drainage were measured as 196.2 ± 61.0 mL (94–352 mL) and 154.4 ± 36.2 mL (85–250 mL). Intraoperative blood loss in group A, group B, and group C was calculated as 177 ± 50 mL, 206 ± 66 mL, and 210 ± 66 mL, respectively. Postoperative drainage was 144 ± 36 mL in group A, 159 ± 36 mL in group B, and 163 ± 40 mL in group C. No significant differences were determined between groups concerning intraoperative and postoperative blood loss (p > 0.05).
Age seemed to have no significant effect on the amount intraoperative or postoperative blood loss and drain removal time. However, BMI and weight of the resected tissue had a strong effect on drain removal time along with intraoperative and postoperative blood loss (p < 0.05).
36.4 Discussion
Many studies have been undertaken to determine the factors that affect surgical and postsurgical bleeding, and some techniques have been introduced to reduce the morbidity of bleeding such as suture techniques [7], local vasoconstricting agents [8–14] and hypotensive anesthesia on bleeding reduction have been reported by many authors [15, 16]. Sex hormone variations may also influence the hemostatic function in female adolescents [17–19]. Some studies showed that contraceptive medications may affect the hemostatic potential in women [20–22]. Sex hormone variations were also thought to be related to the hypercoagulable status during pregnancy [23, 24]. However, there are contradictive results about hemostatic status during the different phases of the menstrual cycle [18]. Some authors found that the levels of von Willebrand factor (VWF) and factor VIII were lowest during menstruation [25–28]. Some other studies reported that there were no cyclic variations in VWF, factor VIII, and factor XI during menstrual cycle [26, 29–32]. Several studies reported on the variations of fibrinogen levels during the menstrual cycle. Some of them reported the lowest levels of fibrinogen during the follicular or midfollicular or luteal phase, while other authors reported no cyclic variation [26, 29–31, 33–39].
High levels of estrogen are believed to be responsible for the proliferation of blood vessels and congestion within in the muscles of the abdominal wall and abdominal skin [40]. Since the estrogen levels change during the menstrual cycle, an influence on bleeding could be expected. It has been shown that the menstrual cycle causes periodic changes in female tissues such as the vagina, breast, oronasal mucosa, conjunctiva, and eustachian tube, which are more sensitive to hormonal changes [41–50].
Ali and Essam [51] reported abdominoplasty combined with Cesarean delivery carries a higher incidence of complications in a study that included 50 pregnant women. They did not report any complication regarding bleeding in their study groups, but they needed to exclude two of the patients from the study and halted the abdominoplasty procedure due to intrapartum uterine atony and bleeding.
Sariguney et al. [1] claimed that blood loss during the perimenstrual period is greater for breast reduction surgeries. Findikcioglu et al. [2] showed that the periovulatory period was detected as the phase in which most bleeding occurs for rhinoplasty patients. However, Findikcioglu et al. also showed that the menstrual period (even menopause) has no effect on intraoperative and postoperative blood loss for operations on sites that are less sensitive to sex hormone levels such as the abdomen, and that is the only study regarding the impact of the menstrual cycle on intraoperative and postoperative bleeding in abdominoplasty patients. Therefore, the results of this study suggest that considering the patient’s menstrual date is not of vital importance regarding perioperative blood loss when planning surgery for areas that are not directly affected by menstrual hormonal changes. Patients can also be informed that it is not necessary to postpone the operation during menstruation in the case of such surgeries.
References
1.
2.
Findikcioglu K, Findikcioglu F, Demirtas Y, Yavuzer R, Ayhan S, Atabay K. Effect of the menstrual cycle on intraoperative bleeding in rhinoplasty patients. Eur J Plast Surg. 2009;32(2):77–81.CrossRef
3.
4.
5.
6.
7.
8.
9.