The Blood Supply of the Skin and Skin Flaps
Geoffrey Ian Taylor
Russell J. Corlett
Mark W. Ashton
Knowledge of the anatomy of the cutaneous arteries and veins is fundamental to the design of skin flaps and incisions. Although detailed studies of these vessels were performed by Manchot,1,2 Spalteholz,3 Pieri,4 Esser,5 and Salmon,6,7 they were published in either German, Italian, or French. In the English-speaking world, little attention was paid to the precise anatomy of the cutaneous vessels so that surgeons designed skin flaps randomly on whatever vessels happened to be in the area, assigning rigid length-to-breath ratios to the flaps. It was not until the last four decades, with the introduction of the microsurgical free skin flap,8,9 the revival of the musculocutaneous flap,10 the description of the fasciocutaneous flap,11,12 and the use of tissue expansion13 and flap prefabrication,14 that surgeons and anatomists have returned to the anatomic dissecting room to search and research the intricacies of the vascular pathways to and from the skin. This has been and still is an exciting period of anatomic renaissance, especially with the emergence of “perforator flaps.”15,16,17,18,19,20,21,22
Although much original data have been provided, there has been a concurrent bewildering explosion of new terms and attempts to classify the cutaneous circulation, often based on flap design rather than vascular anatomy. It is worth stating, however, that many of the “new” flaps, whether island, fascial, neurocutaneous, direct, indirect, axial, random, super, septal, arterial, musculocutaneous, perforator, or otherwise, are each simply the product of a surgical insult inflicted on the same basic vascular pattern that exists throughout the body, though viewed through different eyes. Converse23 stated that “there is no simple and all encompassing system which is suitable for classifying skin flaps.” He went on to state that “it is now generally agreed that the anatomical vascular basis of the flap provides the most accurate approach for classification.” Time has supported the veracity of this statement, emphasized by the recent refocus of attention on the anatomy of the cutaneous perforators as the basis for skin flap design.15,17,18,20,21,22,24,25,26,27
OVERVIEW
The skin is the largest organ of the body. Temperature regulation to maintain homeostasis is one of its major roles. This important function is provided by a rich network of cutaneous arteries and veins, especially in the dermal and subdermal plexi, which supply the sweat glands and allow for heat exchange by convection, conduction, and radiation. Although the cutaneous circulation is rich and vast, the metabolic demands of the skin elements are low so that only a small fraction of the potential cutaneous circulation is necessary for skin viability–a fact that is pertinent to the design and survival of various skin flaps.
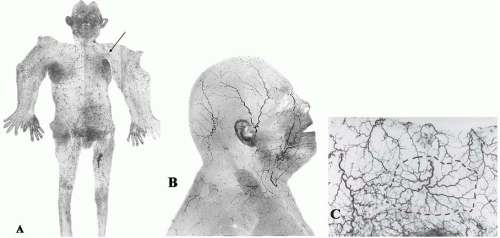
The cutaneous arteries arise directly from the underlying source (segmental or distributing) arteries or indirectly from branches of those source arteries to the deep tissues, especially the muscles (Figures 4.1 and 4.2). From here the cutaneous arteries follow the connective tissue framework of the deep tissues, either between or within the muscles, and course for a variable distance beneath the outer layer of the enveloping “body suit” of deep fascia. They then pierce that structure, usually at fixed skin sites as cutaneous perforators. After emerging from the deep fascia, the arteries course on its superficial surface for a variable distance, supplying branches to it and the deep surface of the fat. They then worm their way between the lobules of the subcutaneous fat, ultimately reaching the subdermal plexus, where they again travel for variable distances to supply the overlying skin, being longest where the skin is mobile.26 During their subcutaneous course, the cutaneous arteries (and veins) often travel with the cutaneous nerves, either as long channels or as a chain-linked system of vessels.28,29
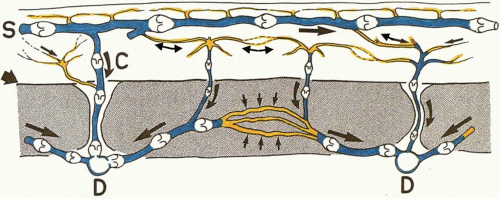
The density, size, and direction of the cutaneous perforators vary from region to region, being modified by growth, differentiation, and the functional demands of the body part, factors that provide the basis for the various anatomic concepts that follow. In general, the vessels of the head, neck, torso, and proximal limbs are larger and more widely spaced than their counterparts in the forearms, legs, hands, and feet (see Figure 4.1). Although the size and length of the cutaneous perforators may vary, they all interconnect to form a three-dimensional “body carpet” that has a particularly well-developed horizontal strata of vessels in the dermis, in the subdermis, on the undersurface of the subcutaneous fat, and on the outer surface of the deep fascia (Figure 4.2).
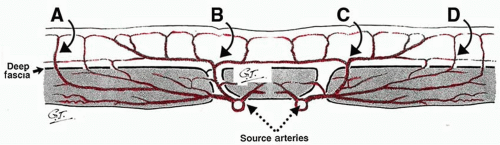
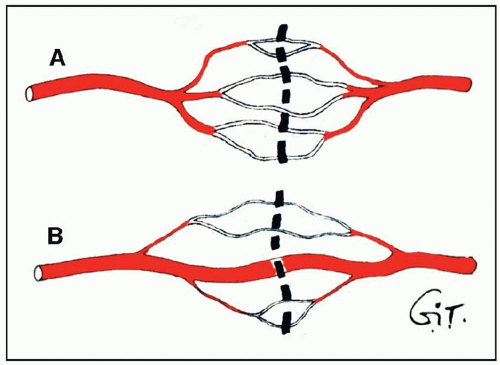
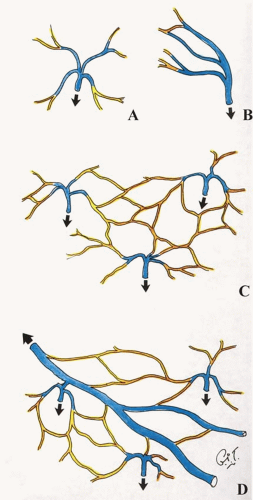
The connections between adjacent cutaneous arteries are either by true anastomoses, without change in caliber, or by reduced-caliber choke anastomotic vessels (Figure 4.3). The latter are plentiful in the integument (skin and subcutaneous tissues) and may be important in regulating the blood flow to the intact skin (Figure 4.1C). These choke vessels play an important role in skin flap survival, where, like resistors in an electrical circuit, they provide an initial resistance to blood flow between the base and the tip of the flap. When a skin flap is delayed by the strategic division of cutaneous perforators along its length, these choke vessels dilate to the dimensions of true anastomoses (see later), thus enhancing the circulation to the distal flap. Although some dilatation of the choke vessels occurs because of the relaxation of sympathetic tone, the major effect is seen between 48 and 72 hours after surgery.30,31 This is due to an active process resulting in hypertrophy and hyperplasia of the elements of the vessel wall and a permanent increase in diameter of its lumen.30
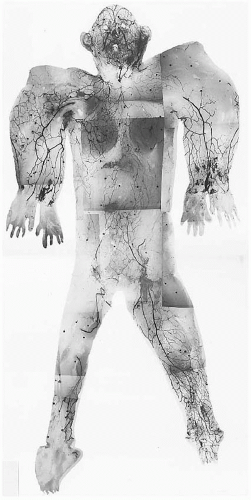
The cutaneous veins also form a three-dimensional plexus of interconnecting channels with dominant strata in the subdermis (Figures 4.4, 4.5, 4.6 and 4.7). Although many of these veins have valves that direct the blood in a particular direction, they are often connected by avalvular veins.32 These avalvular (oscillating) vessels allow bidirectional flow between adjacent venous territories whose valves may be oriented in opposite directions, thus providing for the equilibration of flow and pressure (Figure 4.6). Indeed, there are many veins whose valves direct flow initially in a distal direction, away from the heart, before joining veins whose flow is proximal. The superficial inferior epigastric veins (SIEVs) that drain the lower abdominal integument toward the groin are good examples. In some regions, valved channels direct flow radially away from a plexus of avalvular veins as, for example, in the venous drainage from the vertex of the scalp or the nipple-areolar summit of the breast. In other areas, valved channels direct flow toward a central focus, seen in the groin or in the stellate limbs of the cutaneous perforating veins (Figures 4.4 and 4.6).
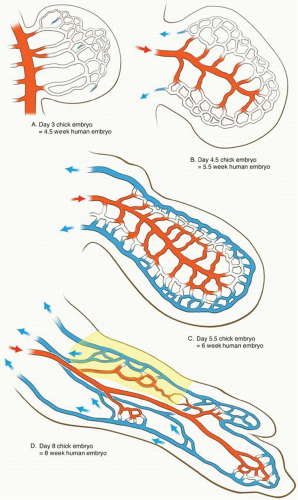
In general, the cutaneous veins partner the arteries. However, the venous drainage of the skin is established in the embryo in two stages, which interconnect but which are separated in time by approximately 1 week of development (Figure 4.5).25,33,34,35
The primary system of veins develops first in the human embryo at about 5 weeks in the subectodermal region and is represented in the adult by large-caliber veins, such as the cephalic, saphenous, and external jugular. These veins course often at some distance from the cutaneous arteries, they are accompanied frequently by cutaneous nerves,28,33,34 and they travel for long distances before piercing the deep fascia (Figures 4.4, 4.5, and 4.6).
The secondary system of veins develops approximately 1 week later in the embryo. This network consists of central axial source veins that accompany the axial source arteries and receive perforating veins from the subectodermal region that accompany the developing cutaneous arteries (Figure 4.5D). In the adult, they are represented by the venae comitantes of the cutaneous perforating arteries with which they travel in close proximity (Figures 4.2 and 4.4, 4.5, 4.6 and 4.7). Thus, from the dermal and subdermal venous plexi, the veins collect into a horizontal “freeway” of large-caliber veins, where they are often related to the cutaneous nerves and a longitudinal system of chain-linked arteries, or alternatively they collect in centripetal or stellate fashion into a common channel that passes vertically down in company with the cutaneous arteries to pierce the deep fascia (Figures 4.6 and 4.7). Thereafter, these perforating veins remain in company with the direct and indirect cutaneous arteries, draining ultimately into the venae comitantes of the source arteries in the deep tissue.
Importantly, these two systems interconnect, especially in the subdermal plexus. This explains why, for example, the radial forearm free flap will survive on either the secondary system of venae comitantes of the radial artery or the primary cephalic or basilic veins.
Thus, the skin is fed and drained by a continuous network of arteries and of veins formed by vessels whose size, shape, density, and direction vary from region to region in the body. The following observations provide for a better understanding of this variation in vessel anatomy.
ANATOMIC CONCEPTS
The Angiosome Concept
A review of the works of Manchot1,2 and Salmon6,7 combined with our own studies of the blood supply to the skin and the underlying deep tissues enabled us to segregate the body anatomically into three-dimensional vascular territories that we named “angiosomes.”26 These three-dimensional anatomic territories are supplied by a source (segmental or distributing) artery and its accompanying vein(s) that span between the skin and the bone (Figures 4.8, 4.9, 4.10, and 4.11). Each angiosome v can be subdivided into matching arteriosomes (arterial territories) and venosomes (venous territories). Initially we described 40 angiosomes, but this was an intentional oversimplification as many of these territories can and have been subdivided further into smaller composite units, for example, the intercostal and lumbar angiosomes, and we took this concept down to the final branches in the vascular tree, which in the skin is the cutaneous perforator (Figure 4.1C).26,29 In the same way, we subdivided the deep tissues, for example, the muscles, into their component anatomical territories.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree