Tendon
James Chang
Shelly Noland
Julie E. Adams
Daniel P. Mass
John G. Seiler III
John S. Taras
Andrew Trueblood
Michael J. Botte
David M. Kalainov
Randon C. Johnson
Anthony J. Lauder
Jennifer Moriatis Wolfe
Flexor Tendon Biology
Flexor tendons I the hand are intrasynovial and are formed by bundles of collagen fascicles that can be subdivided into radial and ulnar halves and volar and dorsal components. The dorsal portion of the tendon receives its blood supply from two vinculae, which are supplied by branches of the radial and ulnar digital arteries. In contrast, the volar portion of the tendon receives minimal direct blood supply. Synovial diffusion allows nutrients to supply the avascular portions of the tendon. The flexor tendons are covered by a thin epitenon layer and run through a tight fibro-osseous sheath in Zone II. This fibro-osseous sheath makes the repair of flexor tendons challenging as adhesion and scar formation can inhibit gliding and hinder postoperative range of motion and rehabilitation.
I. Flexor Tendon Healing
Flexor tendon wound healing can be divided into intrinsic and extrinsic components.
Intrinsic healing involves the tenocytes within the tendon and on the epitenon layer.
Extrinsic healing involves the inflammatory cells and fibroblasts from the overlying sheath. Extrinsic healing may result in the formation of adhesions between the tendon and its sheath, which interferes with the necessary gliding of the tendon through the sheath.
II. Wound Healing
Like other types of wound healing, there are four phases of tendon wound healing: hemostatic (immediate), inflammatory (0 to 7 days), proliferative (2 to 28 days), and remodeling (starting at sixth week).
The hemostatic phase is characterized by vasoconstriction, platelet deposition, and fibrin clot formation.
During the inflammatory phase, inflammatory cells from the surrounding tissues migrate into the area of injury and phagocytize necrotic tissue and clot. There is also deposition of the extracellular matrix including fibronectin, which is used as scaffolding for collagen deposition and vascular ingrowth.
The proliferative phase is characterized by an increase in the number of fibroblasts, collagen deposition, and vascular ingrowth along the collagen/ fibronectin scaffolding.
During the remodeling phase, the strength of the tendon repair increases. Collagen fibers become parallel with uninjured tendons. Throughout the process of tendon healing, active and passive range of motion is necessary to prevent adhesion formation and promote tendon gliding and repair strength.
III. Growth Factors
Multiple studies have been performed to evaluate the molecular basis of tendon healing and to identify potential growth factors that may be involved. These include vascular endothelial growth factor (VEGF), insulinlike growth factor (IGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and transforming growth factor β (TGF-β).
VEGF has a known role in tumor angiogenesis and also promotes angiogenesis in tissues after injury, including tendons. Its mRNA levels peak 7 to 10 days after repair and return to baseline by day 14.
IGF-1 has been found to promote wound healing, and its presence can reverse the negative effects of steroids. It potentiates cell proliferation and thus possibly tenocyte proliferation. It improves tendon healing by stimulating the proliferation of cells and collagen, by decreasing the size of the injured area, and by increasing the strength of the tendon.
bFGF is increased for 8 weeks postrepair of a tendon injury. Its effects include increasing the number of tendon fibroblasts and increasing the amount of collagen type III.
PDGF is expressed very quickly after tissue injury. It plays a role in fibroblast proliferation, collagen deposition, and angiogenesis. It has been found to be increased in healing tendons where it increases the production of collagen, proteoglycan, IGF-1, and DNA synthesis in tenocytes.
TGF-β is a ubiquitous growth factor that is secreted by many cells in the body. It has been found to be increased in tendons and sheaths after injury. It increases collagen production by tenocytes. This causes increased scar formation and fibrosis. Research has investigated the possibility of inhibiting its role to prevent adhesion and scar formation and improve tendon repair. Inhibitors that have been investigated include TGF-b antibodies and the molecules decorin and mannose-6-phosphate. Studies have indicated that the supplementation of decorin and mannose-6-phosphate may reduce TGFb-β-induced collagen production. The intraoperative application of mannose-6-phosphate has been shown to significantly increase postoperative range of motion.
IV. Future Directions
Recent advances in flexor tendon repair and rehabilitation include the use of multistrand repair techniques and early active motion protocols. While these have led to better outcomes in terms of improved repair strength and range of motion, there is still a need for further advances for this difficult problem. An understanding of the biomolecular cascade of growth factors in flexor tendon wound healing will have significant clinical implications. In the future, manipulation of growth factors will allow the acceleration of tendon healing and/or inhibition of excessive healing and adhesion formation.
Acute Flexor Tendon Injuries
Acute injuries to the flexor tendons are common. Treatment and prognosis depend on the location and extent of the injury.
I. Anatomy
Zones:
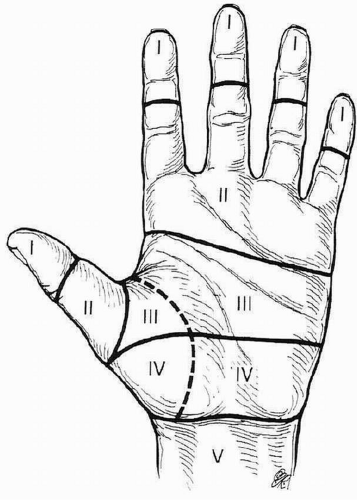
The flexor tendon system of the hand is divided into five zones (Fig. 10.1).
Zone I is the area distal to the flexor digitorum superficialis (FDS) insertion at the mid-middle phalanx. This area is distal to the FDS insertion; thus only the flexor digitorum profundus (FDP) is injured in lacerations within this zone.
Zone II represents the area from the FDS insertion at mid-middle phalanx proximally to the A1 pulley at approximately the level of the distal palmar crease, comprising the proximal portion of the digital sheath. Topographically, this is the area from the distal palmar crease to the midregion of the middle phalanx.
Zone III comprises the area between the proximal aspect of the A1 pulley and the proximal aspect of the origin of the lumbrical muscles from the FDP tendons. Topographically, this represents the region from the distal portion of the transverse carpal ligament (TCL) and the distal palmar crease.
Zone IV represents the region within the carpal tunnel.
Zone V extends from the musculotendinous junction distally to the proximal edge of the TCL.
FDS
The FDS flexes the proximal interphalangeal (PIP) joints.
The median nerve innervates the FDS.
The FDS has two heads of origin.
The ulnar head originates from the medial epicondyle, the medial collateral ligament, and the proximal ulna.
The radial head originates from the proximal radius distal to the supinator insertion.
The FDS courses superficial to the median and ulnar nerves and separates the deep muscle group of the forearm (FDP, flexor pollicis longus [FPL], pronator quadratus) from the superficial layer (pronator teres, flexor carpi radialis [FCR], palmaris longus, flexor carpi ulnaris [FCU]).
In the midforearm, the FDS muscle divides into superficial and deep layers, which give rise to 4 tendons that course under the flexor retinaculum in a specific configuration: The border digit tendons (index and small) run dorsal to the central digit tendons (middle and ring). In 25% of cases, the FDS to the small finger is absent.
FDP
The FDP flexes the distal interphalangeal (DIP) and the PIP joints.
The FDP to the index and middle fingers is innervated by the median nerve, and the FDP to the ring and little fingers is supplied by the ulnar nerve.
The FDP originates from the proximal ulna and interosseous membrane and courses in the deep layer of flexor muscles.
The FDP of the index finger often arises from a separate muscle belly, allowing more independent motion.
The four FDP tendons course deep to the FDS tendons through the flexor retinaculum and the carpal tunnel.
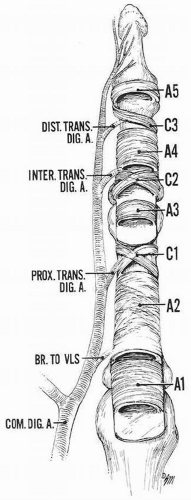
In the digits, the flexor tendons traverse through fibro-osseous sheaths lined with synovium. A series of five annular and three cruciate pulleys are formed from condensations of the sheath.
Five strong annular pulley bands allow for close apposition of the tendon to bone, while the thinner three cruciform pulleys collapse to assist in full digital flexion.
The pulleys are ordered from proximal to distal (Fig. 10.2).
A1 originates from the palmar plate of the metacarpophalangeal (MCP) joint.
A2 arises from the periosteum of the proximal region of the proximal phalanx.
C1 lies between the A2 and A3 pulleys.
A3 originates from the palmar plate of the PIP joint.
C2 lies just between the A3 and A4 pulleys.
A4 originates from the periosteum in the middle of the middle phalanx.
C3 is located between the A4 and A5 pulleys.
A5 originates from the palmar plate of the DIP joint.
At the entrance to the fibro-osseous sheath, the FDS tendons lie superficial to the FDP tendons.
Subsequently, the FDS tendon divides into two slips that wrap around the FDP tendon such that the FDP becomes volar to the FDS as the two slips rejoin via fibers of Camper chiasma (Fig. 10.3).
The FDS slips insert on the mid-middle phalanx, while the FDP continues through the fibro-osseous sheath to insert on the volar base of the distal phalanx.
II. Examination
The patient with a suspected tendon injury should be examined before administering local or regional analgesics to determine the extent of injury. The distal neurovascular status is assessed by two-point discrimination and capillary refill.
The location and extent of laceration are documented, as is the resting posture of the hand and digits. In the presence of a flexor tendon laceration, the digits assume an extended posture at the DIP and PIP joints. Wrist extension does not change this position, in contrast to the tenodesis effect resulting in obligatory finger flexion with wrist extension in the intact hand.

Figure 10.3 FDS tendon divides into two slips that wrap around the FDP tendon such that the FDP becomes volar to the FDS as the two slips rejoin via fibers of Camper chiasma.
The location of injury should be assessed to determine which tendon(s) have been compromised.
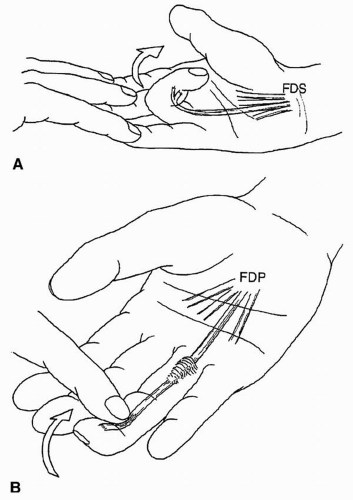
FDS continuity can be tested by examining for isolated PIP flexion of each digit with the other digits held in full extension at the MCP, PIP, and DIP joints. The patient is asked to flex the free digit actively at the PIP joint. Presence of active flexion indicates continuity of the FDS to that digit, although a partial laceration cannot be excluded (Fig. 10.4A).
The continuity of the FDP is assessed by asking the patient to flex the DIP joint while the PIP joint is held in extension (Fig. 10.4B).
Radiographs should be examined to exclude bony injuries.
Treatment is based upon the zone of injury and injured structures. Delayed treatment of injuries is described in the reconstruction section.
III. Zone of Injury
Zone I
Zone I is the area distal to the FDS insertion at the mid-middle phalanx. Because this area is distal to the insertion of the FDS, only the FDP is injured in lacerations of this zone. Avulsion injuries are also possible within Zone I.
A Jersey finger is an avulsion of the tendon from the distal phalanx or from a fracture of the base of the distal phalanx.
Classically the ring finger is involved, and the mechanism of injury is a sudden hyperextension moment applied to a finger with the FDP in maximal contraction.
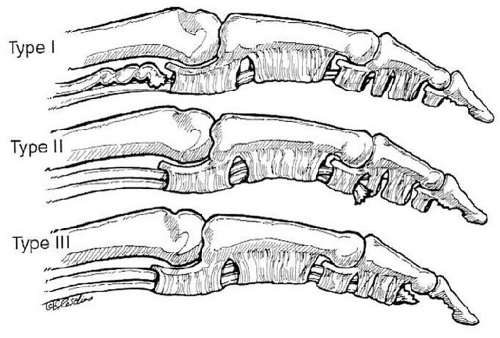
Leddy described three types of Zone I flexor tendon injuries (Fig. 10.5).
Type I: The proximal tendon is retracted into the palm. Disruption of the vincular blood supply and proximal musculotendinous retraction indicate that early repair may be of benefit.
Type II: The tendon is retracted to the PIP joint. Repair is possible for as long as musculotendinous compliance allows.
Type III: Avulsion of the tendon with a bony fragment. The bony fragment usually is entrapped by the distal edge of the A4 pulley, which prevents retraction. Delayed repair is usually possible.
Under brachial tourniquet control in the operating room, the FDP insertion site at the distal phalanx and the A4 pulley are exposed through a volar zigzag or midaxial incision.
The tendon is sutured and is prepared for attachment to bone. The stitch is passed through or around the bone to exit dorsally and distal to the lunula through the nail plate and sterile matrix (to avoid nail growth abnormalities). The ends can then be tied dorsally. Suture anchors have also been used successfully. Larger bony avulsions usually require fixation with pins or screws.
Lacerations
The proximal tendon is exposed either locally in the bed of the wound or retrieved via techniques described for Zone II below. If the tendon retracts proximally, passing the tendon through the A4 pulley may require sequential dilatation of the A4 pulley and trimming of the tendon stump.
If the distal limb of the laceration is less than 1 cm, advancement of the tendon and primary repair to bone may be considered. More than 1 cm of shortening is not advised due to the development of a quadregia effect, and tendon-to-tendon repair is needed. Primary tendon-to-tendon repair is described below.
Zone II
Zone II is the area from the FDS insertion at mid-middle phalanx proximally to the A1 pulley, comprising the digital sheath. Topographically, this is the area from the distal palmar crease to the midregion of the middle phalanx.
Lacerations in this area may involve both the FDS and the FDP. This area is known as, “no man’s land,” as historically results of tendon repair in this region had poor results.
Tendon repair
Empiric data suggest that primary repair be undertaken if more than 60% of the tendon cross-sectional area is disrupted. If less than 60% of the tendon is disrupted, then it may be sufficient to debride the cut edges to prevent tendon catching at the pulleys.
Contraindications to primary repair include tendon disruption in the setting of purulent infection, extensive contamination, or soft tissue deficiency.
Timing of repair may be dictated by concomitant injuries such as vascular insufficiency, but in the setting of an isolated tendon injury, early repair is ideal. Repair can typically be completed up to three weeks following injury. Results of direct repair after a delay in treatment of more than 3 weeks are less satisfactory.
If the repair is not performed emergently, the wound should be irrigated and the skin loosely closed in the emergency department. Appropriate antibiotics and tetanus prophylaxis should be given. The patient should be placed in a dorsal splint.
Repair should be performed under brachial tourniquet control in the operating theatre. If concomitant nerve or vessel injuries are to be repaired, this work should be undertaken before tendon work as the flexed posture of the digit after tendon repair may make further surgery difficult.
The tendon ends are exposed using midlateral incisions or zigzag incisions incorporating the lacerations. The sheath may be opened via radial or ulnarbased flaps between the A2 and A4 pulleys. The annular pulleys should be preserved, but it is often necessary to open the C2 or C1 pulleys.
The tendon ends may be captured and held for repair by 25-gauge needles. This helps to minimize tendon trauma. The ends are minimally debrided to prepare for suturing.
The proximal tendon stump may be visible in the fibro-osseous sheath and can be retrieved using gentle pressure to milk the tendon from proximal to distal. If the tendon has retracted proximally to the palm, then a red rubber catheter tube may be passed retrograde from the wound into the palm. An incision is then made in the palm, and the tube is sutured to the tendons. The tube is pulled back out of the distal wound, carrying the tendons with it.
The distal tendon stumps can usually be delivered into the wound by flexing the DIP and PIP joints. If less than 1 cm of the tendon can be delivered by this technique, it may be necessary to incise the next distal cruciate pulley to gain sufficient working length.
It is critical to restore the anatomic relationship of the FDS and FDP such that the FDP is passed through the FDS slips at the decussation. Repair of the lacerated FDS slips may require smaller sutures (4-0 or 5-0) to decrease trauma to the small slips and reduce bulkiness.
In most cases, repair of both the FDS and the FDP is indicated. In cases where the bulk of the repair does not allow gliding within the flexor sheath, one slip of the FDS can be excised. If the tendon stumps are contaminated or frayed, repair of the FDP alone with excision of the FDS tendon can be considered. Caution should be used in choosing this option because it limits future procedures should repair fail.
Repair strength is proportional to the number of core sutures crossing the repair. Historically, most repairs were performed with 2 core strands, but most authors currently recommend a minimum of 4 core sutures to cross the site. Additional strands of core suture increase repair strength, but must be balanced against the surgeon’s ability to suture the tendon atraumatically and between bulk and gliding of the repaired tendon.
3-0 or 4-0 nonabsorbable braided polyester or fiberwire suture is typically utilized for the core suture.
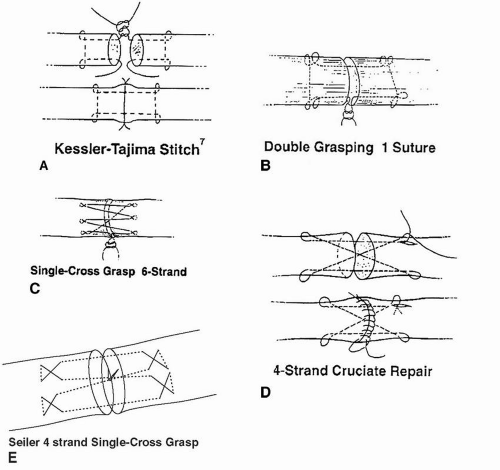
Suture method depends on the surgeon’s preference (Fig. 10.6).
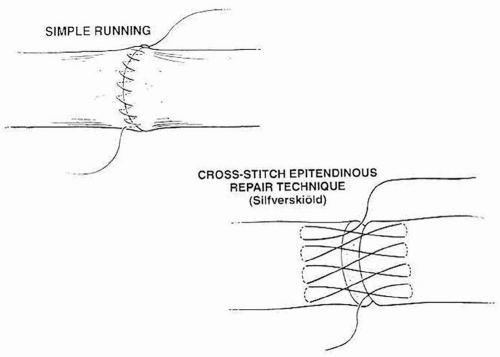
Epitendinous sutures decrease gap formation, improve contour, and improve strength of the construct between 10% and 40% of ultimate tensile strength. Typically, 6-0 running Prolene suture is preferred (Fig. 10.7).
Before closure, tendon gliding through the A2 and A4 pulleys should be assessed with passive flexion. Gentle pulley dilatation as indicated may be required.
The tourniquet is released, hemostasis is obtained, and the wound is closed. The hand should be immobilized with a dorsal plaster slab extending from the forearm to the fingertips of all the digits. The wrist should be positioned in 30 degrees of palmar flexion, the MP joints in 60 degrees of flexion, and the PIP and DIP joints in full extension.
IV. Zone III
Zone III injuries are usually complex, due to the proximity of flexor tendons to other critical structures including the common digital nerves, the superficial arch, the motor branch of the median nerve, and the lumbrical muscles.
Wounds are extended proximally and distally in Bruner fashion; then they are thoroughly explored, irrigated, and debrided.
Injuries to the deep structures of the palm, that is, the deep motor branch of the ulnar nerve and the deep palmar arch, are repaired prior to addressing flexor tendons.
Usually, a lumbrical muscle tethers cut flexor tendons and prevent them from retracting from the wound bed.
If the proximal end cannot be identified, a second longitudinal incision is made in the forearm and the tendon is identified at this level.
A short whip stitch is placed in the proximal tendon end and then used to pass the tendon through the carpal tunnel and into the distal surgical wound.
The tendon is then repaired in the usual fashion.
The decision to repair an injured lumbrical muscle depends on the extent of tissue loss.
Repair without advancement: Repair fosters return to normal function and also improves blood supply to the tendon repair site.
Excessive advancement will result in paradoxical IP joint extension with attempted forceful flexion (lumbrical plus finger).
Following tendon repair, microsurgical repair of superficial neurovascular structures may proceed.
V. Zone IV
Zone IV injuries are rare because of the protection provided by the TCL and carpal bones. Injuries to this depth commonly involve the median nerve, as it is the most superficial structure in the carpal tunnel.
The wound is extended into the palm and forearm, in line with the long axis of the ring finger.
Skin flaps are retracted and the soft tissues are irrigated and debrided.
If possible, cut tendons should be repaired either proximal or distal to the carpal tunnel without releasing the TCL.
In cases where the median nerve is lacerated, however, the carpal tunnel must be fully released.
TCL is step-cut
Begin radially through the proximal ligament, then transversely through the body of the ligament, and completing through the ulnar half of the distal portion.
Hemorrhagic tenosynovium is thoroughly debrided and proximal and distal tendon ends are matched and repaired.
A four-stranded, locked-cruciate core stitch with a running epitendinous stitch is used.
The median nerve should be repaired after all tendons are repaired.
The step-cut TCL ends are then sutured with 3-0 absorbable suture in the Z-lengthened position.
Repair of the TCL is done for prevention of bowstringing during rehabilitation.
In cases where the TCL is irreparable due to injury, the palmaris longus tendon or a long toe extensor may be harvested and used to reconstruct the ligament. The graft is woven through remaining tissue and sutured to itself.
Postoperative rehabilitation is not altered by TCL reconstruction.
VI. Zone V
Acute injuries to flexor tendons and the flexor musculature in Zone V may be repaired within 3 weeks of the time of injury. After 3 weeks, permanent musculotendinous unit shortening occurs and secondary reconstruction or tendon transfers may be required to correct the defect. If the injury is more than 7 days old, the surgeon should also be prepared to perform nerve grafting for median nerve injuries.
The traumatic wound should be extended proximally and distally, and the wound should be carefully explored, irrigated, and debrided.
The presence of hematoma within a tissue plane gives the surgeon an indication of the depth of the wound.
Hemorrhagic synovium in particular should be debrided, examining for injured tendons that may have retracted into the muscle belly.
The volar antebrachial fascia is incised to improve exposure, but the investing fascia of each individual muscle should be preserved whenever possible to facilitate suture repairs.
A muscle will not hold suture, but its fascia will.
The proximal and distal ends of the cut tendons are then matched.
If distal tendon ends have retracted into the carpal tunnel, then the TCL should be released to allow adequate exposure.
The TCL is step-cut, beginning radially through the proximal ligament, continuing transversely through the body of the ligament, and completing through the ulnar half of the distal portion.
The TCL is repaired in a lengthened position at closure to prevent bowstringing of tendons during rehabilitation.
Traction is applied to the distal tendon to identify its corresponding digit.
Due to interconnections between flexor tendons and their synovial sheaths, more than one digit may flex.
The principle digit supplied by a tendon may be identified by comparing the force of flexion exerted at the digital pads during traction.
Four cues are checked to identify the proximal tendons.
First, tendons are provisionally identified by their position in the forearm.
General appearance:
Profundus tendons are flat and multistranded
Superficialis, FPL, and the index finger profundus are oval.
Angles of laceration
Cross-section size and shape
Final check of tendon matching:
Tendon ends are brought to apposition and secured with a transfixing hypodermic needle.
When all tendons are connected, the normal flexion cascade of the fingers should be recreated.
Repair
Profundus tendons are repaired first, followed by the FPL and the superficialis tendons.
Tendon-tendon repair: 3-0 Ethibond (or 3-0 or 4-0 looped Supramid suture) using a locked four-stranded core stitch and the repair site “tidied” with a 6-0 nylon, running epitenon stitch.
Muscle-tendon repair: Interrupted 3-0 Ethibond, Kessler stitch with a running nylon epitendinous stitch.
Intramuscular injury: horizontal mattress stitches with 0 and 2-0 absorbable suture are placed through the investing fascia to approximate the lacerated ends.
After tendon repair, whether in zones III, IV, or V, flexor tendon tension should be uniform and the cascade of resting fingers returned to normal with tenodesis intact. This is the final check before wound closure.
VII. Segmental Tendon Defects in Zones III to V
The advancement of profundus tendons in the presence of a segmental tendon defect should be avoided. In such cases, direct end-to-end repair will result in shortening of the FDP. Because the profundus tendons share a common muscle belly, the shortened tendon will decrease the excursion of the other tendons and thus result in the loss of terminal flexion at all fingers. This is commonly called the “quadrigia” effect. Specific conditions:
End-to-end repair of the profundus is not possible: The distal end is sutured to the adjacent profundus.
End-to-end repair of the superficialis is not possible: Tendon is not repaired and the patient is left with a profundus finger.
Interposition tendon grafts may also be considered to fill in tendon substance defects.
Palmaris and toe extensor tendons are the most commonly used grafts.
VIII. Partial Tendon Laceration
Although the injured tendon maintains some measure of continuity, management of partial lacerations of flexor tendons poses a significant challenge for clinical judgment. Primary concerns are predicting the tendon’s capacity to tolerate loads and avoid late rupture and the prevention of limited range of motion due to tendon entrapment and “triggering.” Secondary rupture is uncommon.
Although repair of partial lacerations of more than 60% of the flexor tendon is commonly advocated, as little as 25% of the flexor tendon may provide adequate strength for unrestricted active range of motion.
Repairs are done in the usual fashion—a core stitch of 3-0 braided suture with a running epitenon repair with 6-0 nylon.
Lacerations involving 33% to 60% can typically be treated with an epitendinous suture only.
Lacerations involving less that one third of the tendon substance are best treated with debridement of the tendon ends. The lowest resistance to gliding results from trimming of the flexor tendon to a smooth contour.
This showed no adverse effects on tendon strength.
Partial sheath excision may also improve tendon gliding in the digital sheath.
The A2 and A4 pulleys should be preserved.
IX. Flexor Tendon Rehabilitation Principles
Despite technical improvements in flexor tendon repair, particularly in Zone II, outcomes are fair to poor in 7% and 20% of cases and secondary surgeries including tenolysis and joint capsular release are often performed. Rupture has been reported in up to 6% of repairs, most often due to poor patient compliance with postoperative limitations but also resulting from technical errors and overly aggressive therapy. Appropriately directed therapy, matched to patient needs and disposition, is crucial for the avoidance of complications and the optimization of outcomes.
Tendon healing (see earlier portion of the chapter on Tendon Biology)
While the extrinsic healing response has the potential for greater and more rapid collagen deposition at the repair site than the intrinsic, it carries the disadvantage of forming restrictive adhesions.
These adhesions limit tendon excursion, resulting in loss of active flexion and/or the loss of differential motion at the PIP and DIP or differential motion between digits.
Activity restrictions must reflect the predicted strength of the tendon repair as it relates to the time from surgery. Tendon repairs weaken for the first 4 weeks after surgery if immobilized, but have been shown to maintain time zero repair site strength for the first 3 weeks postoperatively if mobilized using a clinically relevant place-and-hold synergistic wrist protocol.
At the end of the first postoperative week, repair strength is 50% less than that of the original repair if immobilized. If the tendon is mobilized passively during this time, no loss of time zero repair site strength occurs.
At 3 weeks, strength is decreased by 30% if immobilized. The intact (gap ≤3 mm) repair site begins to accrue ultimate strength beginning at three weeks.
By the end of the sixth postoperative week, the tendon repair is 20% stronger than at the time of surgery. If mobilized using a synergistic wrist protocol, then repair site strength can increase by a factor of two or more during weeks 4 to 6 postoperatively.
Adhesion prevention
Modern rehabilitation protocols are based on the use of controlled mechanical loading and tendon excursion to mechanically inhibit the formation of extrinsic tendon adhesions.
Although up to 9 cm of flexor tendon excursion is needed to achieve full, combined wrist and finger flexion, only 3 to 4 mm of intrasynovial tendon glide is needed to prevent adhesion formation.
Tension in excess of the tendon repair site’s force of gapping may cause gaps in excess of 3 mm, if not overt rupture.
Increased tendon gapping does not directly result in loss of range of motion, but delays the development of tendon strength and may contribute to late tendon rupture as the demands of therapy increase.
There are a variety of rehabilitation protocols used in modern practice. Some place more stress on the tendon repair site than others. Some require greater patient adherence to activity limitations and tolerance for discomfort than others. Careful matching of patient characteristics to the rehabilitation regimen is necessary to optimize patient performance and satisfaction.
Edema control
Tendon motion during the first 5 postoperative days has been shown to increase the tensile strength of repairs when compared with similar tendons treated with immobilization.
Edema in the repaired tendon and its surrounding soft tissues increases the resistance to finger flexion during this same period. The additional work required to flex the digit may exceed the force-to-gapping of the tendon repair, resulting in a weakened or even ruptured tendon.
Recommendation:
Short-term restriction of flexion, lasting no more than 3 days, while aggressive edema control consisting of elevation and digital wrapping is begun.
Extension stretching with the goal of achieving full PIP extension may begin immediately after surgery.
X. Flexor Tendon Rehabilitation Programs
Passive range of motion (Duran protocol)
Passive exercises performed under therapist’s supervision.
Postoperative splint: Extension block splint with IP’s extended, MCPs flexed 70 degrees, and the wrist flexed 10 degrees.
Passive flexion and active extension exercises are allowed in the splint.
Passive extension is allowed in isolated joints, with all other joints in maximal flexion.
At three weeks, the wrist splint is reheated and the wrist is extended to zero degrees.
At one month, “active motion” in the form of place-and-hold exercises is begun.
The digit is placed in a fully flexed position and the patient voluntarily holds it in this position.
At 6 weeks, the patient is weaned from splinting and active motion begins under therapist supervision.
Blocking exercises at the DIP and PIP are used to provide differential flexion between FDS and FDP from this point on.
Ten weeks after surgery, resisted strengthening exercises begin.
All activity restrictions are lifted at 16 weeks.
Kleinert modification of passive motion protocol
Elastic bands are attached to the digit’s nail and pass under a palmar hand pulley to the wrist.
The palmar pulley maximizes DIP and PIP flexion compared to models that attach directly to the wrist.
Active finger flexion is restricted, but fingers are passively flexed by the elastic bands. This maintains a protected position and allows for active extension exercises.
This protocol results in a higher incidence of flexion contractures at the PIP than is seen in the Duran protocol.
Rubber bands should be removed at night and the hand returned to a resting position with the IP’s extended.
Active motion protocols
All controlled motion protocols are designed to provide tendon excursion without the creation of nonfunctional gaps. Passive motion protocols avoid active tensioning of the repair site and instead rely on externally applied forces to produce tendon gliding, preventing adhesions.
Proponents of active motion protocols, however, argue that true proximal migration of the repair site depends on tension from the musculotendinous unit.
Externally applied forces may buckle or roll the tendon during joint motion rather than cause the tendon to glide through its surrounding tissues.
At least 300 g of intrinsic tension is required at the repair site to achieve predictable tendon excursions with passive motion of the digits.
Improved suture materials and techniques using four or more suture strands crossing the repair site have been employed to resist unacceptable gapping and repair site rupture during active motion.
The most commonly employed protocol of this type was popularized by Strickland.
Strickland protocol
Tendon repaired with at least a four-stranded core suture and an epitendinous stitch.
Two splints are employed.
The first is a conventional dorsal blocking splint: IP’s extended, MCPs 50 degrees flexed, and wrist 10 to 15 degrees flexed.
The second splint, a hinged “tenodesis” orthosis, is provided. This allows the wrist to be fixed anywhere from full flexion to 30 degrees of extension, allowing for an increased tendon excursion with synergistic wrist extension and finger flexion. MCPs are maintained in 60 to 70 degrees of flexion to decrease forces on flexor tendons.
Therapy is begun within the first 48 hours after surgery and combines edema control with range of motion exercises.
The patient is then instructed in “place and hold” exercises.
Here, the wrist is extended 30 degrees and the fingers fully flexed passively.
The “hold” component involves the patient actively holding the digit in this flexed position for at least 5 seconds.
When not exercising, the patient returns to their dorsal blocking splint.
After one month, the patient may discontinue use of the tenodesis splint and begins to place and hold at progressively greater MCP extensions.
At 6 weeks, the resting splint is discontinued and active finger flexion begun. Joint blocking at the PIP and DIP increases tendon excursion and favors differential flexor movement.
At two months, light strengthening may be instituted.
Full, unrestricted activity is allowed at 14 weeks.
Early active motion
Postoperative splinting: Patient placed in extension block splint with IPs extended, MCPs flexed 70 degrees, and the wrist extended 20 degrees.
Active extension of the fingers is allowed from the time of surgery.
Controlled active flexion is begun with limited repetitions at 24 to 48 hours and is repeated every four hours throughout the day.
Two repetitions of passive flexion to the palm are followed by two active hold exercises that place the PIP at 30 degrees and the DIP at 5 to 10 degrees of flexion in the first week.
Flexion is advanced gradually to 80 to 90 degrees of PIP flexion and 50 to 60 degrees of DIP flexion by the fourth postoperative week.
Light strengthening begins at 8 weeks.
All postoperative limitations are lifted at 14 weeks.
Zones III to V injuries
Protected motion is needed to optimize results in all zones.
Either passive, active, or early active motion protocols may be employed at the clinician’s discretion. The choice of protocol depends primarily on the predicted strength of the tendon repair and patient’s perceived motivation and ability to understand and comply with activity restrictions.
Loss of differential digital motion is common in zones IV and V, where repaired tendons lie in close proximity to one another and intertendinous adhesions may form.
XI. The Pediatric Patient
Children less than 10 years old and patients with developmental or psychosocial limitations that predict for inability to comply with activity limitations should be casted in 20 degrees of wrist extension and full MCP flexion with fingers fully extended. Immobilization for 3 to 4 weeks is followed by hand therapy to regain active and passive flexion. Tenolysis and/ or surgical release of joint contractures may be needed once the tendon repair matures.
Flexor Tendon Reconstruction
I. Rupture Repair
Repair site rupture can be caused by multiple factors after flexor tendon repair
Patient non-compliance with the aftercare regimen
Non-availability of skilled hand therapy
Formation of peritendinous adhesions
Complex injury
Repair method
Patient assessment
Loss of flexor tone
Assess the flexor cascade and look for loss of appropriate flexor cascade as a sign of repair site rupture. Digits that are swollen can be very difficult to examine.
Loss of tenodesis
If the examiner is skilled, remove the splint and slowly move the wrist from palmar flexion to wrist extension. If the repair is intact and gliding, there should be some increase in digital flexion
Loss of tendon flicker
If the examiner is skilled, remove the splint and ask the patient to demonstrate tendon function with a flicker of active motion
If the examiner is skilled and a multistrand, multigrasp suture method was used to repair the tendon, then place the fingers into flexion, and ask the patient to hold them in that position. Inability to hold the fingers may suggest repair site rupture
Diagnosis
The diagnosis of repair site rupture is based on clinical findings
Ultrasound may be helpful in differentiating adhesion formation from tendon rupture
Early detection of repair site rupture
Assess risks for repair site rupture
Treatment alternatives
Re-repair
Preparation for tendon grafting
Nonsurgical strategies: Range of motion exercises and buddy straps
Late detection of repair site rupture
Assess the risks for repair site rupture
Preparation for tendon grafting
Recover full passive range of motion in both flexion and extension
Allow soft tissues to stabilize
Schedule tendon grafting when the patient has the time and the desire to participate in an intensive hand therapy rehabilitation program
Interphalangeal (IP) joint fusion
Amputation Ray/resection
Nonsurgical strategies: Range of motion exercises and buddy straps
Patient education
Often the results from re-repair are not as good as the results of successful primary tendon repair.
II. Tenolysis
Tenolysis is the procedure that is recommended and performed to remove peritendinous adhesions around the repair site and along the tendon surfaces in order to restore normal tendon gliding and improve digital range of motion.
At 3 to 4 months following tendon repair, patients have recovered approximately 90% of their ultimate range of motion.
Tenolysis can be an effective strategy to improve range of motion when several criteria are met.
The patient has recovered full passive range of motion in both flexion and extension.
The tendon repair is intact.
The soft tissues have stabilized.
The patient is motivated and desires to participate in an intensive therapy program.
Hand therapy is available to the patient.
Operative procedure
The patient should undertake the procedure when they have sufficient time and access to undergo appropriate rehabilitation.
If the procedure is done under IV regional anesthesia, the intraoperative range of motion can be demonstrated to the patient.
Extensile digital exposure is necessary.
The old incision
Midaxial incision may be useful to avoid placing the suture line over the flexor tendon sheath.
Remove all peritendinous adhesions
Initially assess the repair site and remove adhesions from this area.
Secondarily remove any other adhesions that have formed. Small angled beaver blades may be useful for reaching beneath crucial pulleys.
Work within windows in the flexor tendon sheath to restore full tendon excursions.
Manipulate or release any residual joint contracture.
Begin intensive hand therapy on the first postoperative day.
III. Tendon Grafts
Introduction
Tendon grafting is a procedure that uses a free autogenous tendon graft, which is harvested from a distant site, and used to re-establish the continuity of the flexor tendon unit.
Most commonly, the procedure is done to restore the function of the FDP muscle tendon unit.
History of flexor tendon grafting
Until the 1970s, flexor tendon grafting for reconstruction of Zone II injuries was the preferred method of treatment for patients with laceration of the flexor tendons.
With the advent of successful flexor tendon repair, the indications for flexor tendon grafting have substantially decreased.
The results of tendon grafting have improved over time with the introduction of rehabilitative methods that employ digital range of motion and improved
methods for care of associated injuries to the skin, nerve, and skeleton. The choice of the donor tendon may also improve the outcome following tendon grafting.
Indications for surgery
Neglected digital laceration with flexor tendon transection.
Failed flexor tendon repair
Severe crushing injury with segmental tendon loss
Findings on Physical Examination
Loss of normal finger cascade
Absent FDP function on clinical examination
Absent FDS function on clinical examination
Alteration of two-point discrimination on either side of the finger.
Vascular supply of the finger:
Digital Allen testing
Assessment of capillary refill
Assessment of venous congestion
Piability and softness of the skin and subcutaneous tissues.
Pulley function; fixed PIP and DIP flexion deformity if A2 and A4 pulleys are incompetent respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree