35 Technology innovation in plastic surgery
A practical guide for the surgeon innovator
Synopsis
 Plastic surgeons have historically been distinguished by their ability to innovate and should continue to maintain this competitive advantage.
Plastic surgeons have historically been distinguished by their ability to innovate and should continue to maintain this competitive advantage.
 This chapter will familiarize the surgeon-innovator with a systematic approach to innovation. This process includes idea formation, valuation, funding, intellectual property, institutional technology transfer, the FDA regulatory process, and conflicts of interest.
This chapter will familiarize the surgeon-innovator with a systematic approach to innovation. This process includes idea formation, valuation, funding, intellectual property, institutional technology transfer, the FDA regulatory process, and conflicts of interest.
 Negative pressure wound therapy, acellular dermal matrix, and noninvasive body contouring are used to discuss the impact of innovation within plastic and reconstructive surgery.
Negative pressure wound therapy, acellular dermal matrix, and noninvasive body contouring are used to discuss the impact of innovation within plastic and reconstructive surgery.
Introduction
What separates invention from innovation? Invention is the formulation of new ideas for products or processes, while innovation creates the application of new inventions. In business, invention uses cash to create a product and innovation takes a product and creates cash. In medicine, invention is an attempt to create a solution to a clinical problem and innovation drives the solution to the bedside – analogous to the process by which translational research applies basic science to clinical problems.1
Plastic surgeons, by trade, are innovators. We devise innovative solutions to difficult problems. Our competitive advantage lies in innovation. Unlike the neurosurgeon or cardiologist, we do not lay claim to any one part of the body.2 In the hospital we are called upon for our creative solutions to the neurosurgeon’s need for calvarial reconstruction; the cardiac surgeon’s need for chest wall reconstruction, and the orthopedic surgeon’s need for hardware coverage. Our innovative spirit transcends beyond innovative surgical methods and encompasses innovation of novel technologies as well. Historically, microsurgery, distraction osteogenesis, tissue expansion, endoscopic plastic surgery, liposuction and laser technology have all served as platforms to expanding the scope of our practice, as well as expanding the scope of what we can offer our patients.3
Today, however, the arena of medical innovation is far more complex than it was 50 years ago. There is more scrutiny from the US Food and Drug Administration, an exponential growth in the number of patents filed and more complexity in regulatory pathways for new medical products. In the setting of a healthcare and economic crisis, it is harder to justify increased development expenses with increased competition for limited investment funds. When the surgeon is asked to innovate, he/she also faces a plethora of challenges unique to the surgeon innovator including conflict of interest concerns and navigating within the confines of the intellectual property claims of universities.4 The challenges faced by today’s surgeon innovator are captured well by Machiavelli’s The Prince: “There’s nothing more difficult to plan, nor more dubious of success, nor more dangerous to manage than the creation of a new order of things. Whenever his enemies have the ability to attack the innovator, they will do so with a passion of partisans while others defend him sluggishly so that the innovator and his party are likely to be vulnerable.” The enterprise of surgical innovation may receive little support and face many barriers, however it can create new technologies that may revolutionize a field and impact millions of patients. To overcome today’s barrier’s to surgical innovation, we must create and use a systematic approach to translate ideas based on a human problem into a product that can change clinical practice.5 This chapter sets out to discuss a systematic approach to surgical innovation and gives a few examples of new technologies in our field.
The idea
Whether accidental or systemically created, the idea behind an innovation can lead to the development of two broad categories of innovations: a novel method or a novel device. Because the majority of the chapter discusses medical devices, we will briefly discuss the innovation of novel methods. Delos Cosgrove describes his idea for a novel surgical method: “Several years ago, in preparation to perform aortic valve replacement, I found the ascending aorta entirely calcified and both femoral arteries occluded. Recognizing the danger of cannulating either one of these vessels, I raised the patient’s arm to expose the axillary artery, which I used as the cannulation site. The aortic valve replacement was successfully performed.”6 Bruce Lytle expanded this idea and refined the process of cannulating the subclavian artery for this problem. Substantial changes to a surgical intervention are reviewed by the Institutional Review Board (IRB), funded through academic sources, and described in new academic publications and presentations. The surgical community generally shares new methods without recovering royalties for the benefit of patients, even though novel surgical methods can be patented if desired.7
Determining the value
The impact an innovation has on patient care can be described as revolutionary or incremental. A revolutionary innovation has a significant impact on patient care where an incremental innovation has a smaller affect. Consider the revolutionary impact of the endograft for repair of abdominal aortic aneurysms (AAA). The technique of using a transfemoral intraluminal graft for repair of AAA was first published by Balko and associates in 1986, the first published human experience was by Parodi in 1991, and the first device manufactured was designed by Harrison M. Lazarus and developed by Endovascular Technologies Company.8–10 In September 1999, two devices were granted FDA approval for marketing. Now endovascular repair of AAA offers shorter hospital stays, decreased operative mortality and morbidity and an undisputed advantage to patients with multiple or significant comorbidities. On the other hand, consider the reiterations of multiple laparoscopic dissectors. These instruments have been developed in the attempt to improve laparoscopic dissection, but none with a significant impact on patient outcomes or care.
The impact an innovation has on technology can be defined as enabling or refining. An enabling technology is an innovation that serves as a platform for further developments within a field. In 1976, the Fischers introduced the modern era of liposuction, which is now one of the most commonly performed cosmetic surgery procedures.11 This innovation has served as a platform for many further developments including ultrasound and power-assisted liposuction. Both ultrasound and power-assisted liposuction represent refining technologies. A refining technology is an innovation that marginally improves upon available technology and does not lead to a significant technology change.
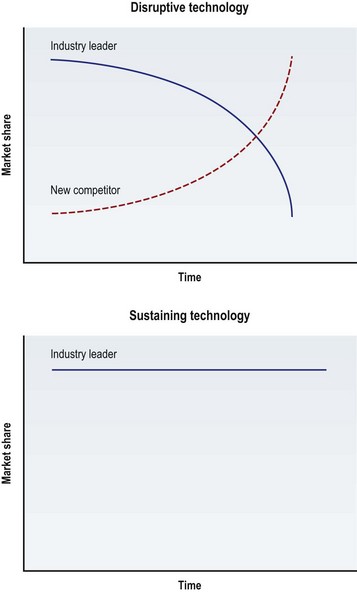
Finally, to help describe the commercial impact of an innovation, the market-based terms “disruptive technology” and “sustaining technology” are used (Fig. 35.1). A disruptive technology is an innovation that supersedes industry leaders and takes over their market share. When disruptive technologies are introduced they are often inferior to the existing leading device and ignored by the incumbent industry leader. In surgery, the device’s inferiority is usually secondary to the device’s learning curve, lack of safety information, and inferior technology. However, as clinicians learn to use the device, as its safety profile expands and as the technology improves, its market share surpasses that of its leading competitor. The “industry leader” can be applied to the corporation that produces the leading technology, or more broadly the subspecialty that uses the technology. For example, when percutaneous transluminal balloon angioplasty was introduced, its safety profile was not well understood and it was inferior to open coronary artery bypass. Over time, it proved to be a disruptive technology that shifted the market share of patients away from cardiothoracic surgeons (the incumbent industry leaders) to interventional cardiologists. The coronary stent, on the other hand, was a sustaining technology. A sustaining technology change is an improvement, usually made by the current industry leader, to maintain growth in the market. The technology can still be enabling (leading to further technology changes) or revolutionary (leading to significant improvements in patient care) but by definition it is not disruptive to market forces. In this case, the coronary stent led to further technologic advancement and improved patient outcomes but did not supersede industrial or clinical leaders. The interventional cardiologist used this technology to maintain their growth in the market.1
Funding
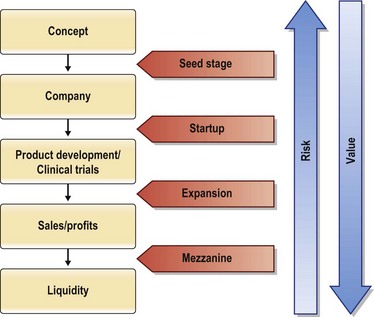
An innovative idea develops from concept, to product, to patient use in a stepwise manner. As each milestone is met, the innovation builds value and reduces risk (Fig. 35.2). Funding throughout this process is acquired based on progress towards building value. The initial smaller investment used to prove the innovative concept is termed seed money. This smaller investment is generally $50 000–$500 000 and can come from a variety of sources: Friends and family, angel investors, device company grants, small business innovation research (SBIR) and technology transfer (STTR) grants. Angel investments come from affluent individuals or a group of individuals, often with industry expertise, usually in exchange for ownership equity in the company. Angel investments in 2009 were estimated at $17.6 billion from 259 480 active investors, with a total of 57 225 entrepreneurial ventures receiving angel funding. Healthcare services and medical devices and equipment accounted for 17% of the investments second only to software, which accounted for 19%.12 Small business technology transfer grants (STTR) reserve a specific percentage of federal R&D funding for partnerships between small businesses and nonprofit research institutions. The idea is to combine the innovative ideas that tend to come out of small businesses or academic centers that lack the means to support serious R&D, with the ability of non-profit research laboratories to develop high-tech innovations. The goal of these partnerships is to transfer the technologies from the laboratory to the marketplace, with small businesses profiting from the commercialization and thus stimulating the US economy. Each year, the Department of Defense, Department of Energy, Department of Health and Human Services, National Aeronautics and Space Administration, and the National Science Foundation are required by STTR to reserve a portion of the R&D funds towards these partnerships.
Intellectual property
To make sure an invention or idea is novel, the surgeon innovator can search the literature and the US Patent and Trademark Office database. One can begin by using keyword searches to find a similar device on any publically available search engines.13,14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree