Chapter 37 Surgical management of complications of burn injury
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Burns and trauma
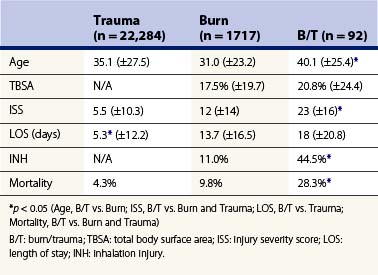
Although the overall incidence of combined burn and traumatic injury is low, the mortality is nearly twice that of burns without associated trauma.1 A retrospective study examined upwards of 24 000 patients with burn, trauma, or combined injuries and found the overall incidence of combined burn and trauma rate to be low (3.8%),2 consistent with the National Trauma Data Bank and National Burn Repository data. Although there was no difference in length of stay, injury severity scores, or mortality among burn or trauma patients alone, there were significant increases in inhalational injury, length of stay, and mortality in patients suffering from combined injuries (Table 37.1). This increase in mortality was seen despite similar TBSA burns, demonstrating the additive effects of trauma and burns in these patients.2 In particular, approximately 24% of military burn injuries are associated with concurrent traumatic injuries, compared to the 2–7% of burn/trauma injuries found in civilians in the same study.3 The most common civilian cause of burn/trauma injury remains motor vehicle collisions (MVC). Of the 500 000 individuals in the United States treated for burn injuries, an estimated 7.2% are secondary to street or highway accidents (American Burn Association, National Burn Repository 2009). MVA victims that require extrication and those that are ejected typically have the most severe multitraumatic injuries. In order of frequency, injured organ systems include musculoskeletal, head and neck, abdominal, thoracic, and genitourinary. Industrial accidents, attempts to escape house fires, explosions, and electrical burns with falls account for the majority of other victims. High-voltage electrical burns rarely occur at ground level and are often accompanied by falls resulting in spinal cord injuries, solid organ injury, intracerebral hemorrhage, and multiple fractures, including vertebral, rib, pelvic, and long bones.
Associated injuries
Vascular injuries may be difficult to diagnose in the presence of burned skin, significant soft tissue edema, compartment syndrome, or hypotension. The principles in their diagnosis remain the same, with an assessment of capillary refill, neurologic deficits, and palpable pulses. A Doppler ultrasound is a non-invasive bedside study that can easily be performed to assess adequacy of arterial blood flow and can be supplemented with an ankle–brachial index (ABI). However, the data, especially the ABI, can be difficult to interpret when extremities are burned full thickness or circumferentially. Although conventional angiography continues to be the gold standard in the diagnosis of major vascular injuries, its benefits do not seem to outweigh its invasive risks in the era of CT angiography. One study found that CT angiography had a >95% sensitivity and specificity in the diagnosis of traumatic arterial injuries of the extremity, with poor arterial opacification being a disadvantage.4 Thus, CT angiography is a reasonable tool in the diagnosis of major vascular injuries. Should a vascular insult require repair, substantial consideration should be given to the choice of graft (autologous versus synthetic), as contamination is not infrequent and only complicates the care of these critical patients. The liberal use of prophylactic muscle flaps for graft coverage has proved helpful.
Gastrointestinal tract complications
Although the superficial effects of burn injuries are often striking, the systemic physiological response to these injuries may result in significant end-organ dysfunction and cannot be overemphasized. Burn injuries >30% TBSA produce a physiological response leading to systemic shock, hypermetabolism, and widespread immunosuppression.5 The combination of intravascular fluid loss, vasoactive hormone release, catabolism, and immune dysfunction results in the non-thermal complications of burn injuries and has consequently become the focus of preventative treatment and molecular models in the design of future therapies.
Physiological changes in blood flow have a dramatic effect on organ response to injury, healing, and permanent dysfunction. This has been particularly well demonstrated in the GI tract, where a combination of diffuse capillary leak, hypovolemia, and the release of vasoconstrictive agents can cause a selective decrease in splanchnic blood flow.6 Splanchnic hypoperfusion occurs early in the post-burn period despite adequate cardiac output and fluid resuscitation, as demonstrated in 40% TBSA pig models that had an early reduction in superior mesenteric blood flow associated with intestinal mucosal hypoxia, acidosis, and increased bacterial translocation.7,8 The GI tract ischemia has multiple effects, including ulcer formation (Curling’s ulcer), enterocolitis, and acalculous cholecystitis secondary to gallbladder ischemia and bile stasis. Other abdominal organs, such as the kidneys and liver, may endure end-organ damage when exposed to the ‘low-flow’ state associated with hypoperfusion following burns, leading to surgical complications and worsened outcomes.
The hypermetabolic response to burn injury plays a key role in non-thermal complications and is characterized by catabolic metabolism, hyperdynamic circulation, insulin resistance, delayed wound healing, while increased risk of infection.9 Poor fibroblast function contributes to impaired wound healing, while leukocyte dysfunction and reduced cellular immunity cause immunological compromise. Alterations in stress hormone responses, cardiac output, lean body mass, and sex hormones may persist for several years post burn. Previous studies have demonstrated typical stress responses to a variety of traumatic injuries, with a so-called ‘ebb’ phase resulting in a decrease in metabolism and tissue perfusion, followed by a ‘flow’ phase 3–5 days following injury.10 Early enteral feeding has been shown to blunt the hypermetabolic response.11
Of all the potential mechanisms of injury, severe burns result in the most dramatic metabolic response. The hypermetabolic phase results in the activation of the hypothalamopituitary axis, sympathetic outflow, the acute-phase response that promotes fat and protein breakdown, and gluconeogenesis.12 Excessive catabolism following a burn injury encourages hyperglycemia and insulin resistance within the first week after injury, both of which are associated with an increase in morbidity and mortality in severely burned patients. Recent trials have demonstrated that intensive insulin therapy aimed at maintaining a daily average glucose of 140 mg/dL improves immune function and reduces sepsis, along with an attenuation of the inflammatory and acute-phase response.13,14 As such, tight glucose control is thought to be critical in improving the overall recovery of burn patients.
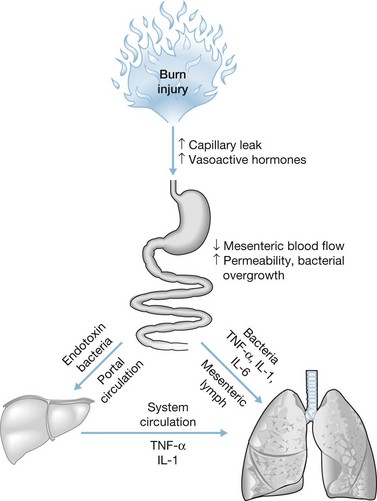
As mentioned, a combination of hypoperfusion and hypermetabolic response can lead to breakdown of the gut mucosal barrier, resulting in bacterial translocation from the gut, systemic inflammation, and ultimately, sepsis (Fig. 37.1). Numerous studies have demonstrated the association between massive cutaneous burn injury and bacterial translocation.8,15 Following massive burn injury, the GI tract mucosa sustains immediate atrophy, resulting in significant gut barrier dysfunction. The increase in intestinal apoptosis does not appear to be from mesenteric hypoperfusion alone, but is speculated to be related to proinflammatory mediators produced by the burn wound.15 An in vivo study using a 30% TBSA burn model in rats confirmed that there was increased bacterial translocation and permeability to macromolecules that peaked at 18 hours post injury, lending credence to the idea that disruption of intestinal barrier integrity in severe burns can lead to sepsis from bacterial translocation.16 Thus, interventions aimed at preventing splanchnic hypoperfusion and hypermetabolism may circumvent complications associated with severe burns.
Recent studies targeting the inflammatory response itself provide insights into potential novel therapeutic targets, such as p38 MAPK, which is involved in the production of the proinflammatory mediators interleukin (IL)-1β, nitric oxide, and cyclooxygenase (COX)-2. Additionally, p38 MAPK is clinically associated with delayed intestinal transit, a common burn complication. Studies inhibiting key steps of the p38 MAPK pathway have demonstrated attenuation of impaired intestinal transit in rodent models, and may provide an innovative therapy for post-burn ileus in the near future.17
Paralytic ileus
Intestinal ileus is a commonly encountered condition following large burns. Multiple factors contribute to the development of ileus in these patients, including sepsis, electrolyte imbalances, narcotic use, and renal failure. Patients typically present with abdominal distension, pain, and/or intolerance to enteral feedings. The cause of the ileus should be thoroughly evaluated on presentation, as it may represent an early indicator of systemic sepsis and can ultimately help direct care. In addition to small intestinal ileus, pseudo-obstruction of the colon, termed Ogilvie’s syndrome, is also a common occurrence in burn patients.18 These patients present in a similar fashion to patients presenting with small intestinal ileus, where abdominal distension accompanied by constipation or diarrhea is frequently encountered. Plain abdominal radiographs demonstrate massive colonic distension.
Treatment is based on the degree of the cecal distension, with the greatest cecal dilation <10 cm treated with saline enemas and/or rectal tube decompression. A persistently dilated cecum over 3 days or estimated cecal dilation >10 cm should be treated with colonoscopy-assisted decompression. Narcotics should be avoided in these patients, as they may worsen the ileus. Recent in vivo animal studies suggest that COX-2 inhibitors may improve delayed gastrointestinal emptying in burn-induced ileus, and thus there may be a potential means of managing post-burn pain without worsening the ileus.19 Operative intervention is rarely necessary, but would consist of a cecostomy or resection followed by diversion.