Skin-Lightening Agents
Marta I. Rendon
Even skin coloration is highly desirable in many cultures and ethnic groups. As a result, hyperpigmentation disorders can cause significant stress and social ostracism.
Hyperpigmentation has many causes (Table 9-1). It can occur as a result of cumulative exposure to ultraviolet light, which can be a factor in melasma, solar lentigines, and ephelides. Hyperpigmentation can also result from certain medications, photosensitizing cosmetics, and inflammatory skin diseases. It can also occur as a postinflammatory response to chemical peels and cosmetic laser resurfacing procedures. In dark-skinned patients, laser therapy and surgical incisions often produce dark-pigmented scars and keloids. Although the incidence of hyperpigmentation occurs with increasing frequency beginning in the middle years, it can occur in young adults and can be associated with pregnancy and treatments for acne.
Multiple skin-lightening agents are available on the market. Many are associated with skin irritation, and an equal number are only partly effective or require long-term use before improvement occurs. In treating patients with darker skin, bleaching agents must be chosen carefully to avoid lightening pigmented lesions beyond the base color or causing irritation, which can lead to postinflammatory hyperpigmentation.
Many skin differences are apparent between individuals with lighter and darker skin tones, as well as among patients in various ethnic groups. These differences affect how the skin reacts to disorders such as melasma and postinflammatory hyperpigmentation, which may be accentuated in darker skin. Ethnic variability must be taken into consideration when treating patients with hyperpigmentation, as darker skin tends to be more sensitive to topical therapy.
Hydroquinone (HQ), a phenolic compound, is the most widely and successfully used agent for a variety of hyperpigmentation conditions, including melasma and postinflammatory hyperpigmentation. Recent interest in natural products has driven research in bleaching agents derived from plant sources and other natural ingredients (Table 9-2). In general, published evidence supporting the efficacy of many of these agents is poor. Fortunately, the effect of some bleaching agents on dark-skinned patients is known from clinical trials and experience. This chapter will include only those agents with evidence of a positive or negative effect on this population.
Table 9-1 Causes of acquired hyperpigmentation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 9-2 Depigmenting agents | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The Melanin Production Pathway and Dark Skin
Knowledge of the melanin production pathway is necessary to understand the mechanism of action of skin-lightening agents. The melanin pathway begins with the action of the enzyme tyrosinase converting the amino acid tyrosine to dihydroxyphenylalanine (DOPA) and then to dopaquinone (see Chapter 2). Dopaquinone is subsequently converted to dopachrome, then to dihydroxyindole or dihydroxyindole-2-carboxylic acid (DHICA). In the presence of dopachrome tautomerase and DHICA oxidase, dopaquinone becomes the brown-black pigment eumelanin. In the presence of cysteine or glutathione, dopaquinone is subsequently converted into the yellow-red pigment pheomelanin.1 Depigmentation can occur when an agent acts on key steps in this pigmentation pathway (Table 9-3).
Agents such as tretinoin act before melanin synthesis, blocking tyrosinase transcription. Many agents are effective during melanin synthesis by blocking tyrosinase or scavenging reactive oxygen species. Others work after melanin synthesis by increasing tyrosinase degradation or inhibiting melanosome transfer, or via accelerating epidermal turnover.1
Table 9-3 Reported effect of depigmentation agents on the melanin synthesis pathway | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Dark or “ethnic” skin generally encompasses Fitzpatrick skin phototypes (SPTs) IV, V, and VI. Variations in skin color depend on various factors, including the amount of melanin produced and the number, size, and aggregation of the melanosomes within the keratinocyte. Although no
racial differences in the total number of melanocytes have been found, SPT VI has a higher total melanin content than SPT I and II (see Chapter 2). Although these differences have certain advantages, including reduced risk of photodamage with enhanced photoprotection and fewer incidents of skin cancer, there are also distinct disadvantages that must be monitored during therapy. These include increased frequency of hyperpigmentation, hypopigmentation, and irritant contact dermatitis. Little research has been conducted on different skin-lightening agents in non-Caucasians with darker skin. Significant data are lacking in reference to efficacy, tolerability, and treatment responses in this patient population.
racial differences in the total number of melanocytes have been found, SPT VI has a higher total melanin content than SPT I and II (see Chapter 2). Although these differences have certain advantages, including reduced risk of photodamage with enhanced photoprotection and fewer incidents of skin cancer, there are also distinct disadvantages that must be monitored during therapy. These include increased frequency of hyperpigmentation, hypopigmentation, and irritant contact dermatitis. Little research has been conducted on different skin-lightening agents in non-Caucasians with darker skin. Significant data are lacking in reference to efficacy, tolerability, and treatment responses in this patient population.
Depigmenting Agents
Hydroquinone
HQ is available over the counter (OTC) in strengths up to 2%, by prescription in strengths of 3% to 4%, and in concentrations up to 10% through compounding pharmacies. Four to 6 weeks of monotherapy with HQ is generally required before depigmentation becomes apparent (Fig. 9-1). HQ is the most potent inhibitor of melanogenesis in vitro and in vivo. It works by competitive inhibition of tyrosinase, which prevents conversion of dopa to melanin. Other mechanisms of action include the inhibition of DNA and RNA synthesis, degradation of melanosomes, and destruction of melanocytes.2,3
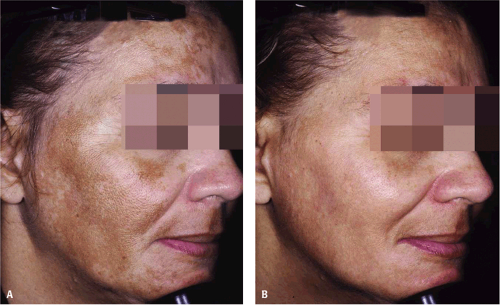
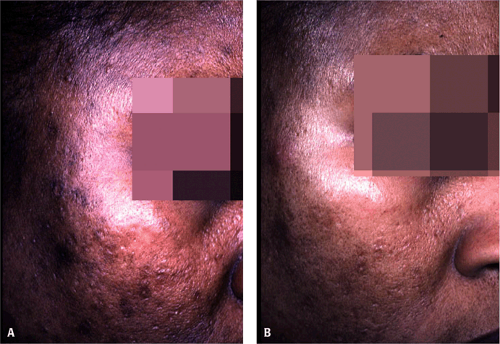
New HQ formulations—combining it with agents such as tretinoin, glycolic acid, vitamin C, retinol, or fluorinated steroids—are now available. The length of time required for these products to take effect varies. However, most patients show some improvement in hyperpigmentation in 1 to 3 months (Fig. 9-2 and Fig. 9-3). The combination of HQ 4%, tretinoin 0.05%, and fluocinolone 0.01% has been shown to have superior efficacy when compared with dyads of HQ and fluocinolone or HQ and tretinoin.4 Several open-label and randomized clinical trials have assessed the efficacy and safety of HQ formulations compared with other modalities or placebo in darker racial ethnic groups. In general, the HQ formulations were well tolerated with minimal side effects.5,6,7,8,9
 Figure 9-1 Melasma. A: Baseline. B: After 8 weeks of treatment with Hydroquinone 4% and retinol 0.15% applied twice daily. (Epiquin Micro). (Courtesy of Pearl E. Grimes, MD.) |
One randomized study examined topical combination therapy with HQ 4%, 0.05% tretinoin, and 0.01% fluocinolone acetonide in blacks, Asians, and Hispanics. No differences were seen in side-effect profile or efficacy among the treatment groups.7
In another randomized, controlled trial of HQ 4% versus placebo (n = 48; 4 men, 44 women) in Brazilian patients ages 19 to 55 with melasma, patients applied the product twice daily along with sunscreen. Outcomes were assessed at 12 weeks by subjective clinical evaluation and photography. Greater improvement was seen in the HQ
group (95%) than in the placebo group (67%), and more patients on HQ (38%) had complete clearance versus those on placebo (8%). No adverse effects of HQ were reported.8
group (95%) than in the placebo group (67%), and more patients on HQ (38%) had complete clearance versus those on placebo (8%). No adverse effects of HQ were reported.8
At this point, it is pertinent to mention that dark-skinned patients often feel they do not need sunscreen and therefore may apply sunscreens only occasionally or at most once daily. Physicians are reminded to address the issue with patients (see Chapter 12).
 Get Clinical Tree app for offline access 
|