Fig. 2.1
Effects of estrogen deficiency
2.3 Estrogen
Estrogen, the main steroid responsible for the secondary gender characteristics in females, affects most systems in the body. The skin is the largest nonreproductive estrogen target area [3].
Estrogen production is regulated by the axle involving the hypothalamus, pituitary gland, and ovary. The pulsatile release of the gonadotropin-releasing hormone from the hypothalamus stimulates the pituitary gland to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In the ovary, LH stimulates the theca cells to produce androstenedione, while FSH stimulates the follicular cells to convert androstenedione to estradiol (Fig. 2.2). The increase in serum estradiol results in the negative feedback to the production of LH and FSH from the hypothalamus, maintaining serum estradiol levels of around 10–20 mIU/mL. Consequently, with a decrease in estradiol production during postmenopause, negative feedback is lost, and there is an increase in serum levels of FSH and LH. The absence of menstruation associated with high levels of FSH and LH is a possible diagnosis of ovarian failure [3].
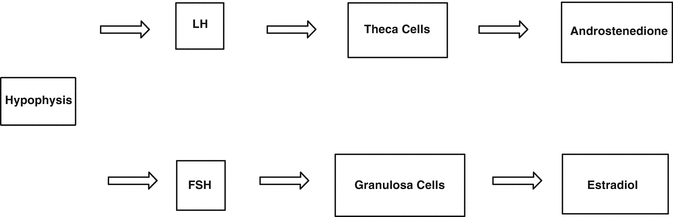

Fig. 2.2
Pituitary axis – the hypothalamus – ovary
2.4 Estrogen and Postmenopause
After postmenopause, estrogen production by the ovaries becomes negligible. However, women maintain detectable levels of estradiol (E2) and estrone (E1) throughout their lives. This occurs as a result of the ability of the peripheral tissues to convert (aromatize) androgens produced by the adrenal glands and ovaries [8, 9].
Estrone is the primary estrogen after postmenopause and is derived from the peripheral conversion of androgens in the muscle, liver, and brain tissue, as well as in the adipose tissue, since the aromatase enzyme displays greater expression in the adipose tissue [8, 9] (Fig. 2.3). Estradiol continues to be produced after postmenopause through the peripheral conversion of estrone; however, it presents in much lower levels than during reproductive life and also in levels lower than those of estrone. The changes in estrogen levels are responsible for most of the morbidity in women after postmenopause, such as osteoporosis, cardiovascular diseases, vulvovaginal atrophy (VVA), and sagging, among others [8, 9].
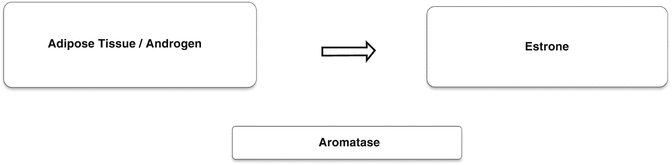

Fig. 2.3
Peripheral conversion of androgen
2.5 Hormone Receptors and Skin
Several functions of the skin have proved to be hormone dependent. Sex steroids clearly play a key role in the skin aging process, as evidenced by the rapid decline in skin appearance noticeable from onset of the premenopausal years. These changes have not been studied thoroughly, although the histological work has demonstrated the presence of estrogen and progesterone receptors in the skin and a relative decrease in expression from the onset of postmenopause [10].
The concentration of estrogen receptors in nuclear and cytoplasmic skin is relatively low. However, surgically induced hypoestrogenemia resulted in a significant decrease in the expression of these receptors [10]. Similarly, studies have revealed a gradient coloration of such specific receptors in the epidermis, with darker color in the granular layer. Similar staining has been observed in the hair follicles and sebaceous glands. Although the presence of estrogen receptors on the skin does not confirm their role on the skin, the even distribution of color suggests that the skin is a major target organ of sex steroids [10].
Based on the levels of the concentrations observed in the female genital tract, the highest concentration of estrogen receptors is found in the vaginal epithelium. Androgen receptors are also expressed on the skin of the external female genitalia. Immunohistochemistry studies identified a greater proportion of estrogen receptors in the vagina compared to androgen receptors, and this ratio in the vulva is reversed where an increased number of androgen receptors are found, with an accompanying decrease of estrogen receptors and progesterone [11] (Fig. 2.4).
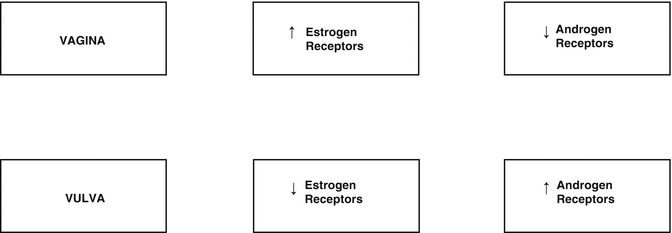

Fig. 2.4
Concentrations of estrogen and androgen receptors in the vagina and vulva
During the postmenopausal period, changes occur in the following indexes; while the concentration of estrogen receptors in the pubic skin remains stable before and after postmenopause, the concentration of androgen receptors, in relation to estrogen receptors, decreases by almost 40 % in the postmenopausal period, and there is also a significant reduction in the level of progesterone receptors [2]. These changes may be relevant for purposes of treatment of dermatoses in the vulvovaginal region in postmenopausal women [10].
2.6 Skin Hydration
The dermis contributes to water retention by way of its hydrophilic content contained in the glycosaminoglycans. The glycosaminoglycans have a negative ionic charge, transporting water to the dermis and, therefore, contributing to skin turgor and tissue protection against excessive compression [12]. Glycosaminoglycans decrease with aging, and this contributes to skin dryness, atrophy, and rhytids [3].
2.7 Skin Thickness and Postmenopause
The thinning of the dermis, clinically recognized by easy bruising and tearing, always occurs with aging. Most studies suggest that the loss of collagen is more closely linked to the postmenopausal period and less to the chronological age, hence hormonal influence [3].
The skin tends to become thinner as postmenopause progresses, and this can be confirmed by high-frequency ultrasound (22.5 MHz). Ultrasound can also be used to measure the thickness of the epidermis and dermis. Several studies using ultrasound have showed that the dermal skin thickness increases with estrogen replacement therapy, such as the finding in a double-blind placebo-controlled randomized study by Maheux et al. The aforementioned study displayed a significant increase in skin thickness, measured at the level of the greater trochanter in postmenopausal women who received conjugated estrogens. This finding was confirmed by both an ultrasound scan and a skin biopsy [14].
2.8 Skin Collagen and Postmenopause
In 1941, Albright et al. observed that postmenopausal women with osteoporosis had a remarkably thin skin, suggesting that the atrophy was more comprehensive than that found in the bone matrix [15]. Brincat et al. demonstrated a similar decrease in the skin thickness and collagen content, corresponding to a reduction of bone mineral density measured throughout the years following postmenopause [15]. In 1983, Brincat et al. concluded in their study that postmenopausal women on hormone replacement therapy with estrogen and testosterone had a collagen content 48 % higher compared to the content measured in untreated women, who were grouped by age [16].
The decrease in collagen levels on the skin occurs at an accelerated rate immediately after postmenopause and subsequently becomes more gradual. Approximately 30 % of skin collagen is lost during the first 5 years after postmenopause, with an average decrease of 2.1 % per year in postmenopausal women over a period of 20 years [10].
2.9 Skin Looseness and Wrinkling
The aging of the skin on the face is characterized by a progressive increase in stretch, associated with reduced elasticity. The loss of tonicity is accompanied by a progressive aggravation of facial wrinkles [17]. Bolognia et al., in a double-blind, placebo-controlled study, observed that young women in early menopause have an accelerated increase in degeneration of elastic fibers in the dermis [18]. Histologically, severe degenerative changes of elastic fibers have been noticed, including the coalescence of cystic spaces into lacunae, peripheral fragmentation, granular degeneration, and the splitting of the fibers into strands. These changes were noticed in individuals 20 years older than said patients. This suggests a close association between estrogen deprivation and changes in elastic fibers [18].
2.10 Wound Healing in Postmenopause
Healing of the skin is first characterized by an inflammation, followed by the formation of granulation tissue, after reepithelialization, and finally, the remodeling of tissues. Delayed wound healing usually occurs in the elderly, and estrogen has been shown to play a crucial role in wound healing [3].
Elastase is capable of degrading a wide range of functional and structural proteins, such as proteoglycans, fibronectin, and collagen. Fibronectin is essential for the healing process by influencing reepithelialization, collagen deposition, and wound contraction. With age, the amount of fibronectin decreases and is degraded secondary by elastase, which is high due to the increased number of neutrophils found in old wounds (Fig. 2.5). Therapies aimed at decreasing elastase, such as those aiming at reducing the number of neutrophils, can enhance the healing process [19, 20].
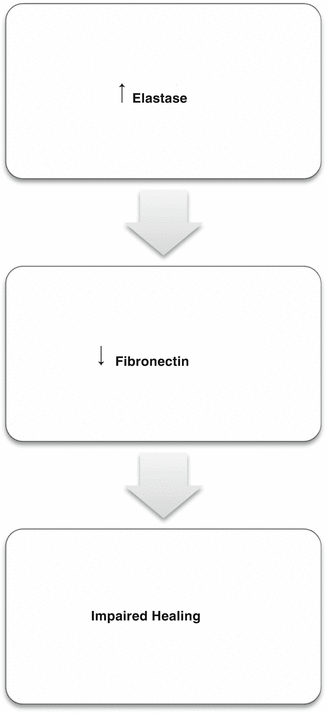

Fig. 2.5
Relation between elastase and healing
Ashcroft et al. in a randomized double-blind placebo-controlled study investigated the effects of topical estrogen on wound healing in healthy elderly men and women and reported the results of this therapy in terms of inflammatory response and elastase levels during the healing process. Compared to placebo, the treatment with estrogen increased the levels of collagen and fibronectin and resulted in a decrease in elastase levels, secondary to a reduction in the number of neutrophils, with consequent reduction in the degradation of fibronectin. The data thus obtained suggest that the delay in wound healing of the elderly can be improved by topical estrogen therapy [21]. Following the same reasoning, recent studies have shown that hormone replacement therapies prevent the onset of ulcers and venous stasis in postmenopausal women [14].
More recently, Ashcroft et al. conducted a study on mice, the results of which suggesting that estrogen also has a role in regulating the migratory inhibitory factor (MIF) of macrophages. Estrogen acts as a proinflammatory agent and has been implicated in the formation of aberrant scarring and in the alteration of inflammatory response in vivo. The estrogen-deficient mice showed a marked increase in the MIF in wound healing, while mice lacking the MIF gene showed no delayed wound healing, despite their estrogen deficiency. We may, therefore, confirm the role of estrogen in the regulation of the MIF and its role in wound healing [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree