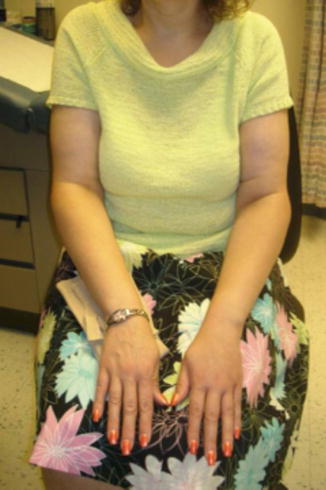
Fig. 8.1
Secondary lymphedema following mastectomy, axillary node dissection, and radiation therapy, complicated by cellulitis. Severe cellulitis, as depicted here, usually requires intravenous antibiotic therapy and possible hospitalization
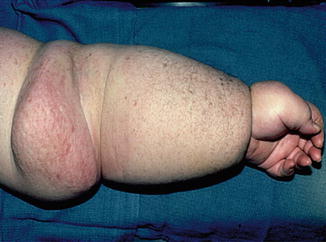
Fig. 8.2
Extreme swelling and skin thickening signifying advanced lymphedema
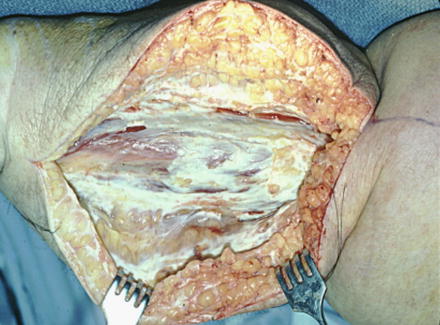
Fig. 8.3
Appearance of the subcutaneous tissues in advanced lymphedema. Note the hypertrophied fat interspersed with a thick, white fibrous tissue. In this degree of thickening, the limb becomes firm to palpation and refractory to compression therapy
Although advanced secondary lymphedema is obvious on clinical examination, the early stages can be less definitively determined, and are often confused with venous dysfunction, other vascular disorders, obesity, lipedema (Figs. 8.4 and 8.5) and various hematological-lymphatic congenital disorders. In a recent review [5] of referrals to a Lymphedema Center, patients were commonly misdiagnosed; only 75 % had a verifiable diagnosis of lymphedema. In 25 % of the group, a diagnosis other than lymphedema accounted for their condition, leading to improper or delayed treatment. Limb volume circumferential measurement along with water displacement devices can assist in the assessment of a correct diagnosis and its severity, but they are not specific for lymphedema. Tape measurements, in particular, are notoriously unreliable because they are so easily influenced by the subjectivity of the strength of pull on the tape or the selection of anatomic landmarks, usually points on bones that are used as end points. Water displacement measurements performed preoperatively and postoperatively assist in an assessment of the efficacy of any surgical intervention. Other techniques under investigation use bioimpedance instruments and infrared laser perometry. Because all of these methods correlate, they can be used collectively in the diagnosis of lymphedema [6–8]. It is clearly difficult to assess edema in certain areas such as the head, neck, breast, and genitalia, especially if circumferential measurements are the mainstay. Patients tend to underreport extremity lymphedema, especially upper extremity edema following breast cancer treatment, either due to the inaccuracy of the method or other issues relating to self-reporting. For those who rely on a determination of upper extremity lymphedema prevalence following breast cancer based on the common instruments described, gross inaccuracies have led to a bewildering spectrum of range, and a confusing picture of how many are actually afflicted.
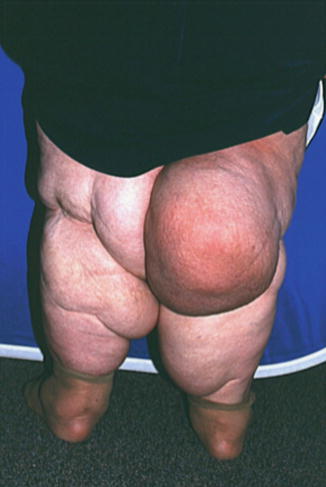
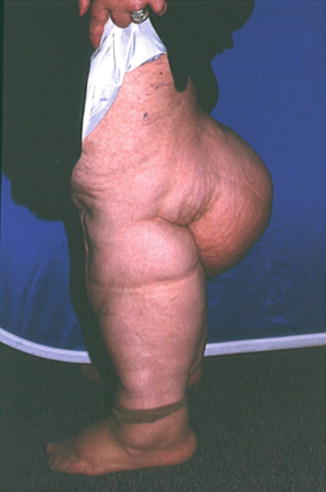

Fig. 8.4
A patient with extreme lipedema. The feet are spared, but extensive deposits of fat are present in both lower extremities above the ankle

Fig. 8.5
In some patients with lipedema, fat deposition in the subcutaneous tissues leads to outpouchings of skin stretched by the accumulated adipose tissue. These extensions create folds of skin which can cause obstruction of the lymphatics localized within them. Areas of permanent lymphedema occur within such regions of a limb with lipedema. A basketball type of appearance usually indicates lymphedema that develops in the context of lipedema, and can be confirmed by lymphoscintigraphy
Diagnostic Imaging
Radiologic lymphography or lymphangiography was popularized by Kinmonth [6] who demonstrated with great clarity the anatomy of the lymphatic system using lipiodol contrast medium. However, toxicity by the contrast agent resulted in damage to lymphatic endothelium and subsequent occlusion of the vessel. Radiocolloid imaging with technetium-labelled antimony sulfide allows lymphoscintigraphic imaging of both lymphatics and nodal basins without toxicity and is virtually free of allergic reaction. Lymphoscintigraphy is not only 100 % specific for delineating lymphatic channels, but can also be used to evaluate lymphatic transport using transit times and demonstrations of lymphatic collateralization and dermal back flow. At our Lymphedema Center, a lymphoscintigram is required in all patients, confirming the correctness of the diagnosis and assisting in adjunctive classification of the degree of lymphatic dysfunction. Failed or markedly delayed nodal visualization documents the severity of the lymphedema; any improvement in the lymphoscintigraphic appearance is extremely useful in the selection of treatment management programs. Lymphoscintigraphy can be used to determine whether certain surgical approaches are indicated, such as a microsurgical or an excisional technique. The status of previously functioning lymphatic channels is an important postoperative finding, helping to validate the efficacy of the operation. Other diagnostic modalities include CT and MRI imaging, but unlike lymphoscintigraphy, are not specific for lymphedema. They do visualize pathologic components of lymphedema, such as hypertrophied fatty collections in the subcutaneous tissues, thickening in the skin, and fluid accumulations that occur in earlier stages with pitting edema, but their low specificity is a problem. Neither MRI nor CT examination is useful in the identification of areas of lymphatic obstruction, a useful observation for new microsurgical constructive techniques of lymph node transfer and lymphatico-venous anastomosis. The most commonly confused conditions—chronic venous insufficiency, lipedema, and obesity—can be differentiated by a combination of physical examination and radionuclide imaging, despite some overlap in these conditions. Lymphedema is not usually associated with chronic venous insufficiency, but such a combination is possible. A lymphoscintigram should be unremarkable in such a patient. Lipedema is distinguished by a number of factors, including bilaterality, female gender association, severity of fat accumulation (most commonly in the lower extremities), absence of iatrogenic causation such as oncological therapeutic intervention, and sparing of the dorsum of the foot when the lower extremity is involved.
Obesity is also distinguishable from secondary lymphedema, but less so with primary lymphedema (which is often bilateral). In extreme obesity [7]—BMI over 40—excess accumulation of adipose tissue causes obstruction of lymphatic by the formation of large skin folds and creases. Lymphoscintigraphy in such patients demonstrates obstruction, dilatation, and extravasation of radiotracer, consistent with a diagnosis of lymphedema. If the obesity persists—the exact timetable is unknown—lymphatic channels can fibrose on a permanent basis. It is also unknown whether effective treatment of obesity with attendant massive weight loss can reverse the lymphatic dysfunction. This risk of obesity to lymphatic function has not been previously appreciated. In addition, procedures associated with massive weight loss surgery such as medial thigh lift can cause secondary lymphedema by injuring lymphatics during the removal of excess skin and fatty tissues.
Secondary Lymphedema and Risk Factors
Swelling of the arm following treatment of breast cancer constitutes the largest category of patients with secondary lymphedema most likely caused by various combinations of surgery and radiation therapy [8, 9]. Specifically, the extent of axillary lymph node dissection has been identified as a major factor, but large population based studies do not exist for confirmation. Lymphedema incidence assessment is complicated by widespread misunderstanding of the definition of lymphedema and its definitive diagnosis, and by major differences in the reporting of secondary lymphedema. Despite using important patient characteristics of extent of surgery, number of nodes, type and duration of postoperative radiotherapy, and the length of follow-up, a wide range of reported incidence, ranging approximately from 2 to 43 %, is known and accepted. When patients report upper extremity lymphedema following breast cancer treatment, an incidence of 14 % based on circumferential measurements is observed; when actual volume measurements are conducted with water displacement and perometry, the incidence of secondary lymphedema of the upper extremity rises to 25.5 %. Lymphedema occurs most often (38 % of patients) after combined axillary sampling and radiotherapy. Commonly accepted risk factors are patient age, body mass index, and length of follow-up. The latter factor adds confusion to the literature because of the wide variation in onset of lymphedema—from a few months to 10–20 years after surgery and radiotherapy. Also, despite the Consensus Document, different gradations of lymphedema severity are used internationally, and many physicians are ignorant of current grading systems.
Some controlled randomized trials have been reported comparing the incidence of lymphedema after sentinel node biopsy with axillary sampling. Not surprising, sentinel node biopsy is associated with less arm swelling than axillary lymph node sampling, which is more extensive and therefore more likely to be by injurious to lymphatic function [10]. Patients reported that the incidence of moderate to severe lymphedema was less with sentinel node biopsy than with axillary sampling, 5 % versus 13 %. However, the reporting was conducted at 12 months after operation, a short follow-up given that most lymphedema occurs later than the first year [10–13]. Patients are usually aware of the risks of axillary sampling, but may not be expecting lymphedema following sentinel node biopsy because it is depicted as a much lesser operation. Unfortunately, secondary lymphedema has been observed in 6.9 % of patients at 6 months and in 5 % at a median of 5 years follow-up. In contrast, axillary lymph node sampling was found to be a cause of lymphedema in 16 % of patients for the same time interval.
Lymphedema remains incurable at this time, and prevention is controversial. Standard precautions include a program of elevation, elastic compression garments, compression pumps, exercise [14], and physiotherapy. Antibiotic therapy is needed as soon as the earliest symptoms and signs of cellulitis become evident: redness, swelling, pain, and fever. Some patients are urged to have antibiotics available immediately at the commencement of an attack, and to seek intravenous antibiotic therapy in a local emergency department. Cellulitis can be unpredictable in the earlier stage of secondary lymphedema. Patients with mild to moderate degrees may avoid attacks of cellulitis completely whereas others will suffer recurrent episodes. Multiple attacks of cellulitis can lead to increasing debility as an ever diminishing quantity of functioning lymphatic channels suffer inflammation, fibrosis, and obliteration of patency. These changes are demonstrable on serial lymphoscintigraphic images, which document the destruction. Patients are advised to avoid blood pressure monitoring and venipuncture in the affected extremity, but there is little if any proof that these precautions are either effective or necessary. The reported incidence of infection following venipuncture in an upper extremity afflicted with lymphedema essentially equals the clean wound infection rate in normal limbs.
Over time, the upper extremity in patients with secondary lymphedema increases in volume from a distal to proximal direction. Pitting edema—present in the early stages, becomes less evident as the high protein fluid in the interstitial space causes progressive inflammation, fibrosis, and fat deposition. Brorson [15–17] was among the first to note that the arm continues to enlarge in some patients, perhaps as part of the natural history of the disease. As a result of adipogenesis stimulated presumably by growth factors within lymphatic fluid—Veg F and others have been identified—a hypertrophy of fat in the subcutaneous compartment containing injured lymphatics and extravastated lymphatic fluid occurs and can continue indefinitely. An interval appears to exist clinically between the onset of lymphedema and the appearance of significant fat hypertrophy, but its duration is unknown. Similar to the mechanism in obese patients, the hypertrophied fat acts to obstruct adjacent lymphatics and abet inflammation and infection. Patients with secondary lymphedema have a 70 times increased risk of infection in the affected versus the unaffected limb.
Secondary upper extremity lymphedema has been associated with post-mastectomy and post-lumpectomy radiation therapy as a result of studies investigating sentinel node and axillary node sampling risks. Upper extremity [18] lymphedema appears to be at greatest risk for women who have both procedures, and the risk continues post-treatment for an indefinite period with this combination. Estimates of lymphedema risk range from 4 to 72 %. With the possible exception of lymphedema occurring in the early weeks or months after axillary dissection and post-lumpectomy radiation, arm lymphedema is not reversible. The chronicity of lymphedema raises the issue of possible malignant transformation over time. Fortunately this risk is extremely low, estimated at .07 to .45 %, but lymphangiosarcoma has been recorded arising in a lymphedematous upper extremity after treatment of breast cancer. When this tumor occurs in a lower extremity following inguinal lymphadenectomy and radiation therapy, prognosis is extremely poor because of the probability of pulmonary metastases. With the limited efficacy of either chemotherapy or radiation therapy, most patients live less than 2 years.
Psychosocial Problems in Secondary Lymphedema
The persistence of lymphedema in the upper extremity has been connected to a multitude of problems involving psychological, sexual, and physical aspects of daily activities. For those in whom the lymphedema develops in the dominant limb, there is greater negative impact and disruption of function. These patients should be supported with appropriate counseling, education, and medications as needed. Frustration, anxiety, and depression are some of the psychological needs to be addressed, along with increased attention directed to covariates of socioeconomic, social, and ethnic factors that determine quality of life (QOL). These factors may be ignored by physicians who, anecdotally, emphasize the sheer importance of “being alive’ over all of the comorbidities associated with secondary lymphedema of the upper extremity.
Secondary Lymphedema of the Lower Extremity
This condition occurs in a manner that is similar to upper extremity lymphedema, but can be more severely debilitating because of the dependency of the lower limb and use in ambulation. Patients with malignancies of the urogenital tract, including prostate and testicular tumors in men and ovarian and uterine malignancies in women are at highest risk, but secondary lower extremity lymphedema has also been reported following trauma or any disease process that injures or destroys inguinal and pelvic nodes. Risk factors mimic those for the arm, including obesity, extent of nodal dissection, duration and intensity of radiotherapy, and characteristics of the tumor.
Scrotal lymphedema, while much less common, is a particularly disabling form of secondary lymphedema that can occur in the same context. Injury to inguinal nodes can obstruct scrotal lymphatics and lead to massive scrotal swelling. There is increased pain from the sheer heaviness of the tissues and much greater risk of cellulitis. The enlargement can result in an appearance of a buried penis. Men can suffer from associated erectile dysfunction and social embarrassment.
Management of Secondary Lower Extremity Lymphedema
The overwhelming majority of cases of secondary leg lymphedema can be managed successfully with compression therapy, including custom-fitted, double layered garments, combined with daily pneumatic compression pumping. Surgical intervention has been reserved for those patients who fail conservative therapy as manifested by increasing bouts of cellulitis and worsening of swelling. Surgical intervention is indicated when conservative therapy fails, with suction assisted lipectomy (liposuctioning) considered to be the first choice technique. Popularized by Brorson (Figs. 8.6, 8.7, 8.8, 8.9, 8.10, 8.11, and 8.12) and others [19, 21], the technique is based on the observation that excess fat in the subcutaneous space appears to be secondary to hyperplasia and adipogenesis. Suction lipectomy is not initiated until the pitting edema has been successfully eradicated by conservative treatment. Once that goal has been accomplished, complete reduction of the limb is achieved by liposuctioning. The patient is required to wear a compression garment custom-fitted for the limb continuously. Follow-up of 11 years demonstrated a complete maintenance of normal leg volume in the involved leg. A significant reduction of morbidity was also seen following liposuctioning for secondary lower extremity lymphedema: frequency of episodes of cellulitis was reduced as were infection related hospitalizations. Patients continue after liposuctioning with exercises, elevation, compression garments, pumps, manual lymphatic drainage, and proper skin care practices.