6
Seborrheic Dermatitis
David E. Castillo1, Ilana Gunczler1, Katlein França2,3, and Jonette Keri1,4
1 Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, Miami, FL, USA
2 Department of Dermatology & Cutaneous Surgery; Department of Psychiatry & Behavioral Sciences, Institute for Bioethics & Health Policy, University of Miami Miller School of Medicine, Miami, FL, USA
3 Centro Studi per la Ricerca Multipdisciplinare e Rigenerativa, Università degli Studi Guglielmo Marconii, Rome, Italy
4 Dermatology Service, Miami VA Hospital, Miami, FL, USA
Introduction and Epidemiology
Seborrheic dermatitis (SD) is among the most common dermatological disorders worldwide. It is a chronic, relapsing, and inflammatory skin disorder with a predilection for sebaceous gland‐rich regions including the scalp, face, and back. SD is considered a multifactorial disorder with three main pathogenic factors contributing to the disease: Malassezia colonization, sebaceous gland activity, and individual susceptibility and immune response [1]. SD is a clinical diagnosis established through history and physical examination; a skin biopsy is rarely needed but can be useful in uncertain cases [2]. Although there are many treatments for SD, none can provide a definite cure, and recurrences are very common. The most common therapies used include inhibition of skin yeast colonization, reduction of pruritus and erythema, loosening of the crusts and scales, and diminishing inflammation. These therapies consist of antifungal agents, corticosteroids, immunomodulators, and keratolytics.
SD is estimated to affect around 11% of the general population [3], with a peak incidence during the first three months of life and between the fourth and sixth decades. The infantile form is self‐limited, and reports have shown an estimated incidence up to 40% [4, 5]. Adult SD have an incidence of around 1–3% in immunocompetent adults and classically presents as a chronic relapsing condition [1, 6]. Men are affected more than women and no genetic predisposition has been established for SD [7].
SD is more common in immunocompromised patients such as HIV/AIDS patients [8–11], organ transplant recipients [12], hepatitis C infection [13], and malignancies [1]. The incidence has been shown to be up to 83% [1, 8], and these patients present a more severe and extensive form of SD, with high rates of treatment resistance.
SD is also more common and severe in patients with neuropsychiatric disorders such as Parkinson’s disease [14, 15], tardive dyskinesia [1], epilepsy, facial nerve palsy [5], and mood disorders [16]. Genetic disorders are also associated with high rates of SD, including Down syndrome [17], among others.
Pathogenesis
Although SD has a high prevalence and psychosocial impact in the society, its pathophysiology is not completely understood. It is considered a multifactorial disorder and three main pathogenic factors contribute to the disease: Malassezia colonization, sebaceous gland activity, and individual susceptibility and immune response.
Malassezia Colonization
Malassezia spp. is a lipophilic, dimorphic fungus that is a normal component of the skin flora, but predominates in seborrheic areas of the body (scalp, neck, chest) [5]. The role of the fungus in SD is still controversial, but a few mechanisms have been proposed. Malassezia spp. is frequently isolated from lesions of SD [18]. It is proposed both that SD lesions have higher numbers of the fungi when compared to healthy skin, and that there is a direct correlation between the number of fungus and severity of the disease [19–21]. However, a few studies have refuted this association by showing no difference in the total number of fungi in affected skin compared to healthy skin [22–24]. Furthermore, a study conducted in HIV/AIDS individuals showed that HIV‐infected patients with SD do not have a higher fungi count than HIV‐infected patients without SD [24]. Although the evidence seems contradictory, the fact that antifungal agents are effective in the treatment of SD suggests that Malassezia spp. plays an important pathogenic role [22, 25]. The fungus has been shown to invade the stratum corneum and to release lipases that break triglycerides which results in local inflammation with abnormal keratinocyte differentiation, and hyperproliferation, and subsequent hyperparakeratosis [2, 26, 27]. This leads to an abnormal epidermal barrier function with subsequent inflammatory response and the resulting signs and symptoms of SD.
Sebaceous Gland Activity
SD is associated with sebaceous gland overproduction. Characteristically, sebaceous gland production is activated by maternal androgens at birth and continues for a few weeks [7, 28]. After puberty, circulating androgens reactivate sebum production which is maintained until the sixth decade [5, 29]. This correlates with the time frame of SD, which rarely occurs before puberty and is most common during adolescence and adulthood when sebum production is at maximum [6]. Also, SD occurs in the seborrheic areas of the body (active sebaceous glands) including the face, scalp, and trunk. However, it is well known that not all patients with SD have sebum overproduction [7]. Skin‐surface lipid composition plays an important role in the pathogenesis. It seems that patients with SD have high levels of triglycerides and low levels of free fatty acids and squalene [30]. Free fatty acids have anti‐microbial properties, which might explain some of the effects of abnormal lipid composition. Triglycerides are degraded into free fatty acids by lipases released by bacterial skin flora such as Propionibacterium acnes. P. acnes was shown to be reduced in patients with SD [19], which suggests that abnormal skin flora also contributes to the development of SD [7].
Immune Response
It has been proposed that abnormal host immune response plays a role in SD. This is supported by the fact that SD is more common and severe in immunosuppressed patients, especially HIV/AIDS individuals. Several studies have tested this hypothesis, although results have not been conclusive. Some studies have found increased production of proinflammatory and regulatory cytokines such as IL‐1, IL‐1b, IL‐2, IL‐4, IL‐8, TNF‐α, IF‐γ, and histamine in skin samples of patients with SD compared to healthy skin [31, 32]. A few studies have detected high levels of IgA and IgG in patients with SD [31, 33]. However, other studies suggested that the increased antibody titers were not against the Malassezia yeast, but to metabolites secondary to lipase activity or toxin production [34, 35]. This supports the theory that the lipase activity of the yeast triggers the inflammatory skin response that contributes to SD. Regarding cellular immune response, evidence is also contradictory, and studies have not been able to establish if there is an increase or decrease in the CD4+/CD8+ T cell ratio [33, 36, 37]. Finally, recent evidence highlights the role of oxidative stress (OS) in SD pathophysiology [38].
Other Factors
The pathogenesis of SD involves several factors. Epidermal barrier function abnormalities due to abnormal lipid distribution and corneocyte function have been described [39, 40]. An impaired barrier allows Malassezia spp. to penetrate the skin and start an inflammatory response. There is a direct link between neurologic disorders and SD. Although patients with Parkinson’s disease present with a more severe form of SD, it is proposed that the underlying mechanism is endocrine rather than neurotropic, as patients with unilateral parkinsonism can develop bilateral SD [1]. There is evidence for a direct link between traumatic brain injury, spinal cord injury, facial palsy, and SD [41]. Interestingly, some recent evidence suggests that genetics play a role in the development of SD, especially to explain some of the factors that lead to individual susceptibility, such as epidermal barrier defects [5].
Drug‐related seborrheic‐like dermatitis can be seen in patients treated with anti‐cancer drugs such as cetuximab, dasatinib, erlotinib, 5‐fluorouracil, sorafenib, interferon α, sorafenib, sunitinib, thalidomide, and vemurafenib, among others [42–48]. Other medications that can worsen or induce SD include griseofulvin, cimetidine, lithium, buspirone, chlorpromazine, ethionamide, haloperidol, methyldopa, thiothixene, phenothiazines, methoxsalen, psoralens, stanozolol, and gold [41, 49]. Although other mechanisms have been studied, including nutritional deficiencies (pyridoxine, zinc, niacin, and riboflavin), none of them can explain this disorder by itself; it is rather a combination of multiple factors that increase individual susceptibility and lead to SD (see Table 6.1).
Table 6.1 Pathogenic factors of seborrheic dermatitis.
| Malassezia colonization |
| Sebaceous gland activity |
| Immune response (Host immune response, HIV infection) |
| Genetic factors |
| Neurologic disorders (Parkinson’s disease, traumatic brain injury) |
| Medications (anti‐cancer drugs, anti‐psychotics) |
| Nutritional deficiencies |
HIV: human immunodeficiency virus.
Psychological Aspects
Skin diseases can severely affect the psychological and social functioning of patients. Several studies have demonstrated the psychological impact of chronic skin disorders and their association with depression, anxiety, and poorer quality of life [50–53]. Despite being a mild disorder, SD is chronic and relapsing, and often impacts psychosocial functioning and quality of life. A few studies showed that the impact of SD on quality of life is comparable to fungal infections and acne, and that it is greater in women and younger highly educated individuals [54–56]. This is likely because these specific populations are more aware of their image. Some authors have even suggested that SD can predispose to depression [57]. It can lead to low self‐esteem and anxiety. Therefore, dermatologists must be aware of the emotional aspects of patients with this chronic skin condition. They must conduct a basic psychological evaluation to determine mental status and to provide reassurance. Patients’ expectations and concerns must be addressed by the dermatologist, as this will help build a trustful doctor–patient relationship, which is a keystone of everyday medical practice.
Clinical Presentation
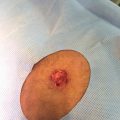
SD presents clinically as well‐demarcated, erythematous plaques with greasy‐looking, yellowish scales distributed on areas rich in sebaceous glands. Most commonly it affects the scalp, the face, and upper chest [2, 358–61]. There is a large variation in extent and morphologic characteristics of the disease, depending on areas affected and age of incidence [61]. SD has a seasonal pattern, presenting more frequently during winter and improving usually during summer. Also, worsening of SD has been associated with stress and sleep deprivation [1, 5, 61].
In children, SD occurs most frequently in the first three months of life. Scaling generally involves the scalp and can affect the face and body folds (retro‐auricular area, neck, axilla, and groin). The infantile form is commonly self‐limited and disappears by the first year of age. Cradle cap is the most common manifestation and appears as an accumulation of yellowish, greasy scales on the scalp [2, 58, 59, 61, 62]. In body folds, lesions have a moist, glistening, non‐scaly aspect and tend to be confluent. Rarely, SD may present as an erythrodermic eruption in infants or as Leiner disease. Leiner disease is a poorly defined entity that is associated with immunodeficiency, generalized exfoliative dermatitis, diarrhea, and failure to thrive [5, 61].
In adults, SD is a chronic and relapsing condition that affects sebum‐rich areas such as the scalp, the external ear, the center of the face, the upper part of the trunk, and the intertriginous areas [1, 2, 5, 58, 61]. It may first appear shortly after puberty or later, peaking between the ages of 40 and 60 years [2, 58, 59, 63].
The clinical appearances involving the scalp differ markedly between patients. The milder and most common form is scalp SD, also known as dandruff; it is restricted to the scalp and presents as fine, white‐yellow diffuse skin flaking without erythema. More severe forms of scalp SD present with visible inflammation and may reach into forehead and retro‐auricular areas [60, 64]. Clinical features range from mild‐moderate desquamation to severe erythematous patches with yellow, greasy‐looking scales and honey colored crusts attached to scalp and hair [5, 62]. SD‐derived hair can be thinner, with a more brittle surface and less shine, and SD can be associated with increased rates of hair loss [26]. Pruritus is not an obligatory feature but is often present [59, 61]. As SD appears on the face, it tends to affect the lateral sides of the nose, the nasolabial folds, upper lip, eyelids, and eyebrows. Involvement of eyelids can lead to blepharoconjunctivitis, which consists of a honey‐colored crust on eyelids with free margin, and can be associated with skin involvement [65]. In men with a beard, mustache, or sideburns, lesions may involve hair‐bearing areas and can improve if the areas are shaved [5, 61].
SD is more common and more severe in the immunosuppressed population; these include organ‐transplant recipients, lymphoma patients, and HIV‐infected individuals, particularly those with CD4 counts below 400 cells per millimeter [59, 60, 66]. In patients with HIV, the lesions are more extensive, markedly inflamed, have atypical distribution (involving trunk and extremities), and are usually refractory to treatment [3, 58, 62, 67]. However, some can have SD regression with highly active antiretroviral therapy (HAART).
Complications of SD include secondary bacterial infections, dermatophyte infections, skin atrophy with prolonged use of steroids and hair thinning due to excessive scratching.
Diagnosis
SD is a clinical diagnosis, based on history and physical examination. The diagnosis can be made based on the characteristic presentation and distribution of lesions and varying course of the disease [2, 61]. The differential diagnosis for SD is extensive and depends on the patient’s age, area involved, and race or ethnic group. The main differential diagnoses includes psoriasis, tinea capitis, atopic dermatitis, rosacea, contact dermatitis, and systemic lupus erythematous (SLE) (Table 6.2) [3, 58, 61].
Table 6.2 Differential diagnosis of seborrheic dermatitis.
Source: Information adapted from [2, 5, 41, 58, 60, 61, 68].
| Common disorders | Distinguishing features |
| Atopic Dermatitis | Appears after 3 mo of age. Flexural lichenification in adults; facial and extensor involvement in children. Pruritus and irritability are common. Personal or family history of atopy. |
| Tinea capitis | Dermatophyte infection of the scalp; scaly patches associated with alopecia with “black dots” (broken hairs). Most common in children. |
| Psoriasis | Thick plaques sharply limited with silvery white scales. Involves nails and extensor, palmar, and plantar areas. Uncommon in children. |
| Contact Dermatitis | Polymorphous features including erythema, edema, and vesicles in acute phase and lichenification and hyperkeratosis in the chronic stage. Irritant diaper dermatitis is confined to the diaper area and in contrast to SD spares the skin folds. |
| Rosacea | Affects malar area of face; desquamation is atypical; may be associated with telangiectasia and recurrent flushing. |
| Systemic lupus erythematous (SLE) | In the acute stage the “butterfly” rash has a bilateral malar distribution, spares nasolabial folds and nose bridge; photosensitivity is common; usually is associated with other clinical signs of SLE. |
| Erythrasma | Well‐demarcated erythematous patches on intertriginous areas, lesions are stable and asymptomatic; bright coral‐red fluorescence on illumination with Wood’s lamp |
| Pityriasis versicolor | Hyperpigmented or hypopigmented, round to oval skin lesions that are most commonly found on the seborrheic areas of the body. Can be associated with pruritus. Most common in adolescents and adults. |
| Rare disorders | |
| Langerhans’s cell histiocytosis | Skin/brown colored papules, scales, and crusts on scalp, lesions tend to coalesce and become tender; palmoplantar and nail involvement can occur. Multisystem condition including osteolytic bone lesions and diabetes insipidus. |
| Secondary syphilis | Copper‐colored scaly plaques on palms and soles with peripheral lymphadenopathy. VDRL/RPR, FTA‐ABS* confirm diagnosis. |
| Pityriasis rosea | Salmon/pink colored papules over trunk and proximal extremities in “Christmas‐tree” distribution known as herald patch. |
| Pemphigus foliaceous | Erythema, scaling, and crusting appear on the scalp and face that expands to trunk and back. Histology and direct immunofluorescence with anti‐desmoglein antibodies confirm diagnosis. |
VDRL, Venereal Disease Research Laboratory; RPR, Rapid Plasma Regain; FTA‐ABS, Fluorescent Treponemal Antibody‐Absorption.
In children, SD is commonly misdiagnosed with atopic dermatitis and tinea capitis [2]. Atopic dermatitis has a later onset than SD, usually appearing after the third month of life and affecting extensor areas, which are rarely involved in SD. Also, atopic dermatitis patients have a personal or family history of atopy such as eczema, allergic rhinitis, or asthma. Tinea capitis, a contagious dermatophyte infection, clinically presents as scaly scalp dermatitis with moderate or minimal inflammation; hair loss is generally present with broken hairs seen as “black dots” in the physical exam [61].
In adults, a chief differential diagnostic problem is the distinction of SD with psoriasis; while areas of involvement are similar in both conditions, psoriasis plaques tend to be thicker, sharply demarcated, and have silvery white scales. Also, psoriasis can be associated with arthritis and commonly affects the nails and the extensor, palmar, and plantar surfaces of the skin. Furthermore, both conditions can occasionally coexist [68, 69].
Because of similarities in distribution, SD can be easily misdiagnosed as rosacea but the latter usually involves the cheeks, nose, and eyes; in rosacea desquamation is atypical and may be associated with telangiectasias and recurrent flushing, characteristics not associated with SD [3, 61].
Another common disorder that can be mistaken for SD is SLE. The skin lesions in SLE’s acute stage have a bilateral malar distribution similar to SD, but it spares the nasolabial folds and has a clear photodistribution. Also, SLE is associated with extracutaneous manifestations such as arthritis, hepatobiliary disease, glomerulonephritis, and cardiomyopathy. Histology and serologic tests such as antinuclear autoantibodies confirm the diagnosis [61].
A skin biopsy is rarely needed for diagnosis, but can be useful in rare cases. Histologically, SD can be divided into acute and chronic stages. In the acute stage, SD shows superficial perivascular and perifollicular inflammatory infiltrates, composed mainly of lymphocytes and histiocytes [7]. In chronic lesions, marked psoriasiform hyperplasia and parakeratosis can be present with dilation of the venules of surface plexus, which resembles psoriasis [7, 69]. However, in psoriasis parakeratosis is often associated with thinning or loss of the granular layer due to accelerated keratinocyte differentiation.
Treatment
Although SD is a chronic, relapsing condition without a cure, there are several options to reduce signs and symptoms. These therapies target the main pathogenic factors which are Malassezia colonization and local inflammation. Topical antifungal and corticosteroids agents are the most widely used: however, new emerging therapies such as calcineurin inhibitors have shown to be safe and effective.
Scalp Seborrheic Dermatitis
Mild forms of scalp SD/dandruff are treated with antifungal or anti‐inflammatory shampoos. Ketoconazole 2% shampoo, an imidazole that impairs fungal cell membrane synthesis and has mild anti‐inflammatory properties, is effective in the treatment of scalp SD when applied twice or more a week for four weeks [70–72]. This shampoo formulation can also be used once a week as prophylaxis to prevent relapses once remission is achieved [25, 73]. Miconazole nitrate 2% shampoo, Bifonazole 1% ointment, and fluconazole 2% shampoo are also effective and well tolerated in scalp SD [1, 74, 75]. Ciclopirox 1% shampoo is another antifungal agent with effects against Malassezia spp. It is effective when used two times a week for a four‐week period, and can also be used for long‐term maintenance if used once a week [76–78]. Both ketoconazole and ciclopirox shampoos are safe and well tolerated.
Selenium sulfide exhibits both antifungal and anti‐inflammatory effects [1]. Zinc pyrithione inhibits fungal growth by promoting copper influx and inactivation of iron‐sulfur proteins [79]. Both selenium sulfide 2.5% shampoo and zinc pyrithione 1% are effective for scalp SD [1, 25]. However, selenium sulfide has more side effects, such as hair discoloration and alopecia. Other shampoos containing salicylic acid, lactoferrin, piroctone olamine, and ciclopiroxolamine can be used as adjunctive therapy for scalp SD [80].
Topical corticosteroids are also first‐line therapy for scalp SD. Clobetasol propionate 0.05% shampoo used two times a week for four weeks is effective in cases of moderate scalp SD [81]. Better results are achieved when Clobetasol 0.05% shampoo twice a week is alternated with ketoconazole 2% shampoo twice a week over a four‐week period [82]. Fluocinolone 0.01% solution or shampoo and betamethasone valerate 0.12% foam are also available options for SD [2, 83, 84].
Topical solutions, sprays, or foams with corticosteroids or other agents such as propylene glycol, lactic acid, or urea are useful adjuvant therapies to accelerate the resolution of signs and symptoms of scalp SD [80]. Indeed, recently two randomized, double‐blind trials (RCT) showed a significant improvement of scalp erythema and desquamation with a topical solution with urea, lactic acid, and propylene glycol [85].
Non‐scalp Seborrheic Dermatitis
Infantile SD most frequently resolves with the use of emollients and frequent bathing [7]. Shampoos are safely used in the case of scalp SD. In cases of more severe SD, topical ketoconazole 2% cream, with or without initial low‐potency corticosteroid cream can be safely used [7, 86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree