Picture 17.1
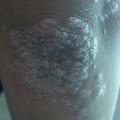
A 32-year old male patient with seborrheic dermatitis in the scalp
The incidence of SD has been shown to be increased in special populations, specifically those who are immunocompromised (especially patients with HIV/AIDS) and those with Parkinsonism or other neurologic disease [4, 5]. In the HIV/AIDS population the incidence increases as high as 83 %, depending on the population studied [4, 15, 16]. The incidence is not only increased but also a more diffuse and inflammatory form of SD has been described in patients with HIV/AIDS [15, 17]. There is some debate as to whether the severity of SD correlates with severity of HIV-1 infection [18–20]. Still, the association of SD in HIV/AIDS is significant: of the HIV-patients studied in an infectious disease clinic in 2010, 98 % were impacted by some dermatologic finding, the second most common (31 %) being SD [21]. Associations between SD and Parkinson’s disease have been linked to increased sebum excretion ratios, increased yeast density, as well as high phosphatase and lipase activity in a recent laboratory-based study [22]. Other reported factors include increased levels of circulating melanocytic stimulating hormone and decreased motility of the face [23, 24]. There is an interesting connection to neurologic stress and SD with reports describing unilateral SD following nerve lesions [25, 26]. SD has also been linked to familial amyloidotic polyneuropathy and Trisomy 21 [27, 28]. Few cases of SD as a paraneoplastic syndrome have also been described [29–32].
SD may be an undesired side-effect following treatment with psoralen and ultraviolet A light [33]. SD has also been associated with erlotinib and sorafenib use [2].
Seborrheic Dermatitis and Stress
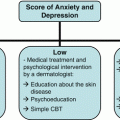
The cause of SD is multifactorial but incompletely understood. Endogenous factors such as lipids, hormones, and the host immune system as well as exogenous factors including seasonality and the skin flora, in particular Malassezia species, have been discussed [4, 34]. A recurring factor in the appearance of SD episodes has been the psychological status of the patient: stress, anxiety, and emotion have been suggested and described as triggering flares [7, 14, 35–38]. Stress and psychological comorbidities have commonly been accepted to be linked with exacerbations of dermatologic disease, including SD [35, 38–40]. Likewise, dermatologic disease has been known to negatively impact quality of life [40, 41]. Formal studies of the link between SD and stress are few. A study of 2159 patients with SD found that the most common clinical profile included a history of stress, depression, or fatigue prior to the flare 76.4 % (P < .0001) [7]. In a study of 82 patients, Misery et al. found that stress was reported as the main triggering factor, whether it be the initial outbreak or a flare [37]. The stress associated with these flares was more associated with anxiety than depression; however, based on the Beck Depression Index Score, patients with facial involvement were more depressed [37]. Öztas et al. reported increased dermatology life quality index (DLQI) scores compared to healthy controls in patients with SD and suggested that SD may predispose patients to depression [36]. In a recent study by Araya et al., 28.3 % of participants identified emotional stress as a trigger for their SD outbreak [14]. Further, using the DLQI, Araya et al. report that SD has a moderate impact on patient quality of life, however, of note 3.6 % of patients reported an extreme affect; embarrassment was among the greatest complaints [14].
Conclusion
SD is a common dermatologic disease with a varying clinical course and degree of severity. The link between stress and SD, both as an exacerbating factor and as a consequence of the disease, should be carefully considered when caring for patients. Management of both patients’ psychological wellbeing and skin will have a potential synergistic effect on prognosis. Special attention should be given to patients with life-altering comorbidities in order to anticipate SD flares and to aid in the patients’ ability to maintain a positive and realistic outlook. It is highly suggested to inform all patients that SD is likely to recur and that ongoing treatment may be necessary. Ultimately, more well designed, large studies are required to better understand the relationship between stress and SD, and how co-management of the mind and the skin can impact prognosis.
References
1.
Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44–9.PubMedPubMedCentral
2.
3.
Berk T, Scheinfeld N. Seborrheic dermatitis. Pharm Ther. 2010;35:348–52.
4.
5.
Schwartz RA, Janusz CA, Janniger CK. Seborrheic dermatitis: an overview. Am Fam Physician. 2006;74:125–30.PubMed
6.
Johnson MT, Roberts J. Skin conditions and related need for medical care among persons 1-74 years. United States, 1971–1974. Vital Health Stat 11. 1978:i–v, 1–72.
7.
8.
Janniger CK. Infantile seborrheic dermatitis: an approach to cradle cap. Cutis. 1993;51:233–5.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree