Sclerosing Solutions
 INTRODUCTION
INTRODUCTION
Although it might appear deceptively simple, it is not always easy to sclerose a varicose vein. Even a small amount of damage will produce intravascular thrombus, but thrombosis alone usually does not obliterate the vessel. Intact endothelium contains tissue plasminogen activator that can dissolve thrombus. A thrombosed vessel with intact endothelium will simply recanalize.
Vascular fibrosis and obliteration only occur in response to irreversible endothelial cellular destruction. If an injected sclerosant is too weak, the vessel may initially close, but recanalization will occur and an incompetent pathway for reflux then persists. If the injected sclerosant is too strong, the varicose vessel endothelium is destroyed, but the sclerosant will also affect adjacent normal vessels causing damage there as well. The goal is to deliver a minimum volume and concentration of sclerosant that will cause irreversible damage to the endothelium of the abnormal vessel to be sclerosed, while leaving adjacent normal vessels unaffected.
No single sclerosing agent can provide perfect results in every clinical setting. There are not only significant differences between agents of different classes, but there are also important subtle differences between agents of the same class that at first glance seem very similar. There is also a great deal of individual variability in response to sclerosing solutions. The clinician must be prepared to work with a variety of agents to meet the needs of circumstance. The choice of which sclerosing agent to use is based on a good understanding of the mechanism of action of each sclerosing solution.
Virtually, any foreign substance can cause venous endothelial damage. Historical methods for producing venous endothelial trauma have included “a slender rod of iron” (reportedly used by Hippocrates himself), phenol, absolute alcohol, ferric chloride, anti-syphilitic mercurial drugs, and hundreds of other agents. These early sclerosing agents caused many deaths in addition to a high incidence of local tissue necrosis, pain, failed sclerosis, and allergic reactions.
An ideal sclerosing solution would have no systemic toxicity. It would produce local endothelial destruction extending all the way to the adventitia with a minimum of thrombus formation. Because all sclerosants eventually flow into the deep system, an ideal sclerosant would be rapidly inactivated by dilution. It would require a long period of contact to be effective, making it more effective in areas of stasis and safer in normal veins where there is a high flow. It would be nonallergenic. It would be strong enough to sclerose even the largest vessels, yet it would produce no local tissue injury if extravasated. It would not cause staining or scarring, nor telangiectatic matting. It would be perfectly soluble in normal saline. It would be painless upon injection, inexpensive, and approved by the US Food and Drug Administration (FDA).
The ideal sclerosant does not yet exist, but modern sclerosants can exhibit near-ideal behavior when used correctly. The degree of endothelial cell destruction can often be tempered by adjusting the concentration of the sclerosing solution.1,2 Proper patient positioning allows the varicose veins to be treated in a nearly “empty” condition so that a “minimum effective concentration” will disrupt the varicose veins but will be diluted below the threshold of bioactivity immediately upon flowing into the deep veins. The type of sclerosant can be carefully matched to the type, size, and location of the vessel, and the volume used can be adjusted for optimum effect.
Many currently available sclerosants will cause cutaneous necrosis if extravasated. Even those that do not cause extravasation necrosis can still cause cutaneous necrosis if inadvertently injected into a telangiectasia that participates in an arteriovenous anastomosis. Sclerosant flow into the small end-arteriole causes immediate and persistent blanching of all the tissues in a roughly circular area. The problem is largely avoided by using very small injection volumes and slow injection rates under low pressure. If the typical pattern of blanching is observed, the local application of topical nitroglycerine paste has been reported to prevent or mitigate the ulceration.3
 SCLEROSANT CATEGORIES
SCLEROSANT CATEGORIES
Sclerosing solutions are often artificially classified into three broad categories: detergent sclerosants, hyperosmotic agents, and chemical irritants or chemical “corrosives” or “toxins.” All of these agents interfere with the function of endothelial and subendothelial cell surface proteins that are necessary for survival of the cell, but each member of each class does so in a slightly different way. Table 14-1 lists the classification of some commonly available sclerosing agents. Each category of solution has its own properties (Table 14-2). As a reference, there are numerous tables in this chapter to allow rapid comparison of a variety of issues concerning sclerosing solutions (Tables 14-3–14-6).
TABLE 14-1
Classification of Common Sclerosing Solutions

TABLE 14-2
Properties of Sclerosing Solutions
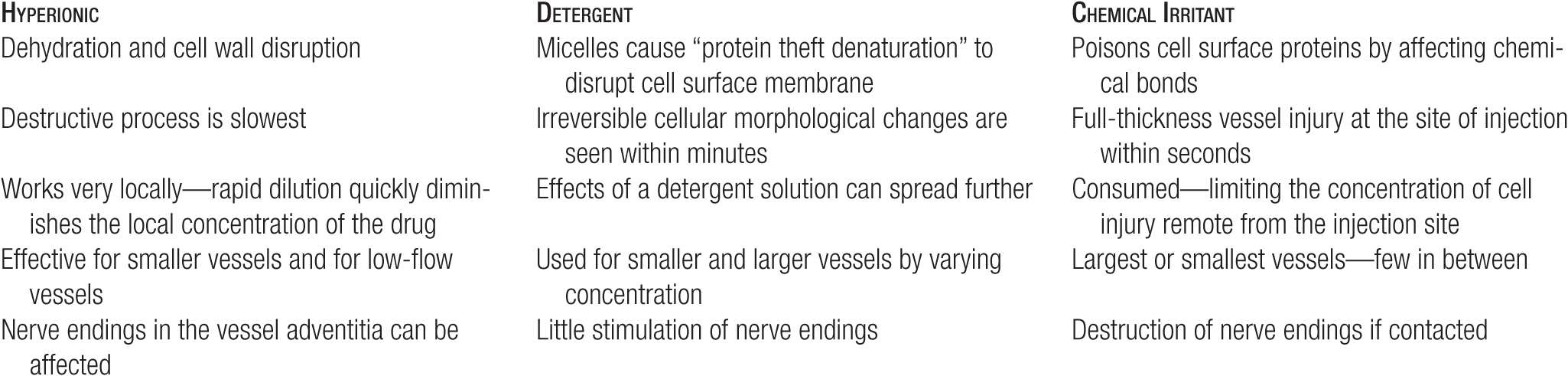
TABLE 14-3
Relative Potency of Sclerosing Solutions (Minimal Sclerosant Concentrations)
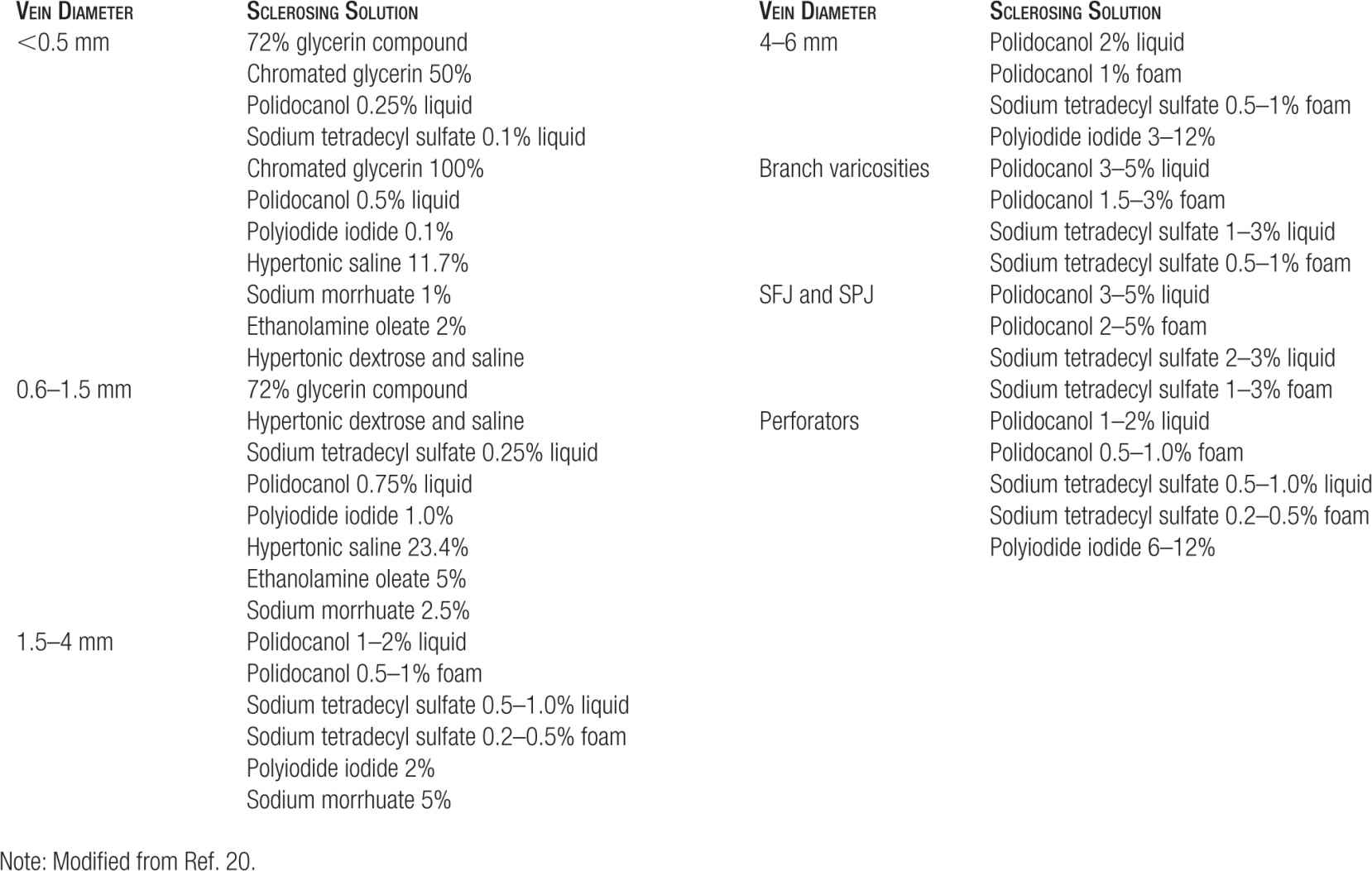
TABLE 14-4
Starting Doses for Sclerosing Agents (Per Point of Injection)
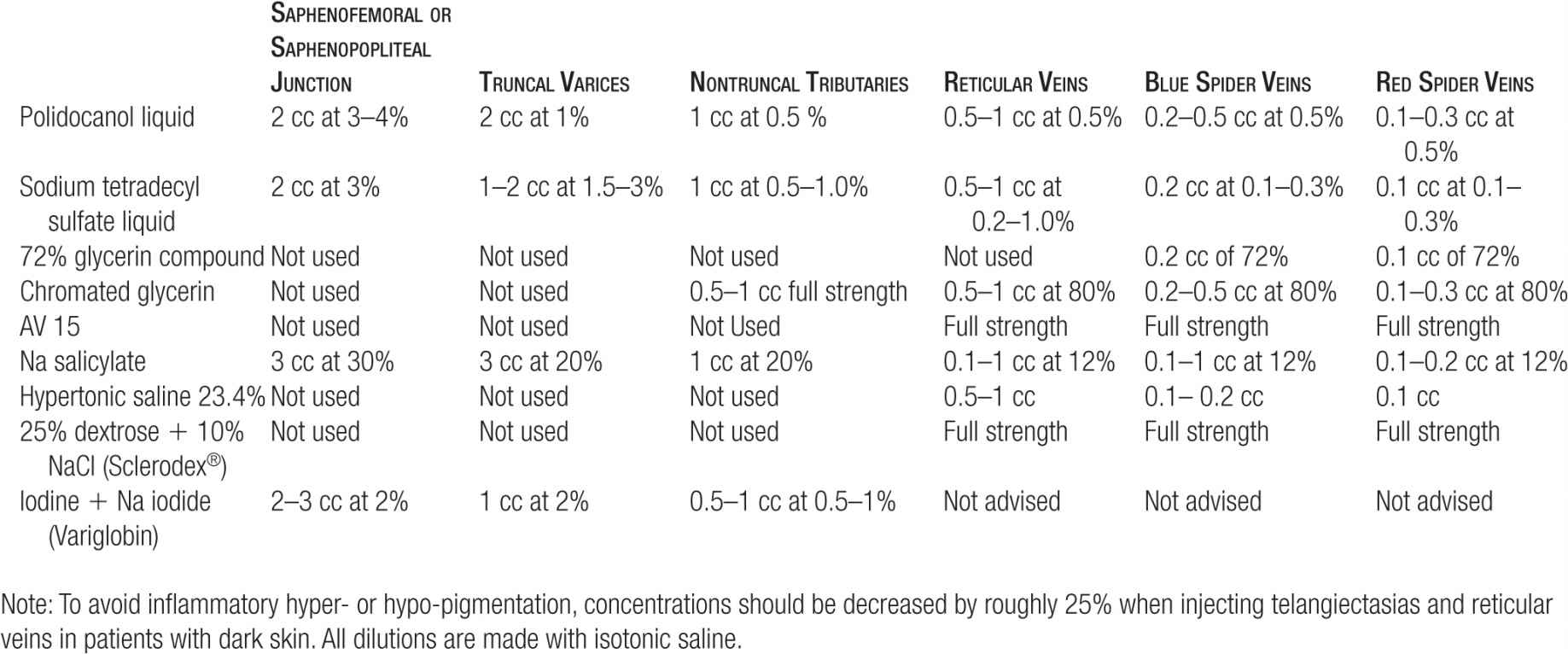
TABLE 14-5
Maximum Doses for Sclerosing Agents (Per Session)
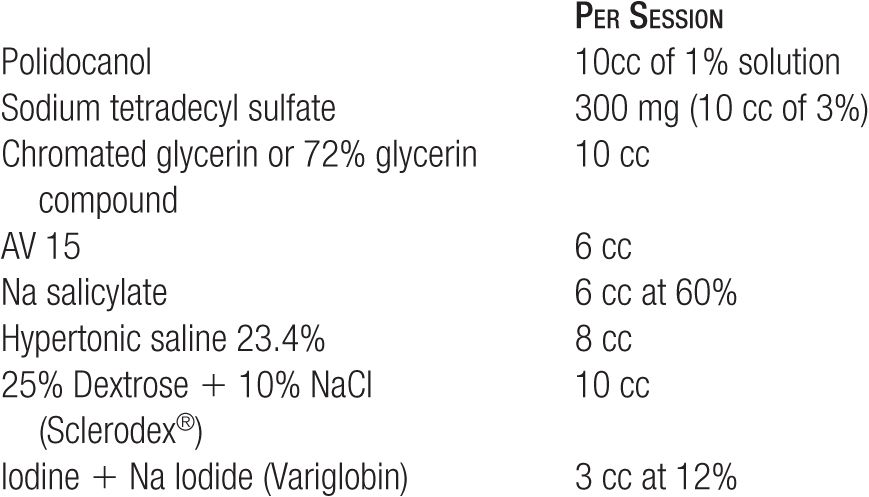
TABLE 14-6
Summary of Primary Sclerosing Agents
Detergent Solutions
The most common detergent solutions utilized in sclerotherapy are sodium tetradecyl sulfate (STS) and polidocanol (POL). Long-chain fatty alcohol detergents with the right combination of lipophilic and polar hydrophilic regions have long been used in biochemistry labs to extract cell surface proteins from otherwise intact cells in vitro. This is most likely the mechanism by which the detergent sclerosing solutions work in vivo, and has been called protein theft denaturation.
For each detergent sclerosant, there is some threshold concentration below which the agent causes no injury. At low concentrations, most detergent molecules are individually dissolved in solution. When the concentration reaches a threshold known as the critical micellar concentration (CMC), nearly all further detergent molecules added to the solution will enter into amphophilic bilayers known as micelles. Individual detergent molecules have no toxicity to the vascular endothelium, but micelles can cause protein theft denaturation and can disrupt the cell surface membrane in other ways.
The loss of essential cell surface proteins causes delayed cell death. When vascular endothelium is exposed to detergent micelles, irreversible cellular morphological changes are seen within minutes by scanning electron microscopy, but the fatal cellular changes that are visible by normal light microscopy do not become apparent for many hours.4 Unlike many other agents, the detergent sclerosants do not cause hemolysis nor do they provoke direct intravascular coagulation.
As a practical matter, the effects of a detergent solution can spread further than the effects of osmotic solutions because it takes more time for bioactive aggregates to dissolve than for the osmotic gradient to disperse. The volume and concentration of these solutions must be carefully tailored to the situation. Varying the strength of the detergent solution and the amount of time the solution is trapped within the vessel changes the timing and the extent of damage.
Detergent sclerosants are potent agents that have a clearcut threshold below which they have absolutely no injurious effect on venous endothelium. Foaming of these agents is easily achieved with agitation and addition of a gas such as air. Foaming the solution allows more efficient sclerosis with exposure to far less sclerosing solution, as the foam can block flow into and out of the treated site.5
Hyperosmotic Solutions
Hyperosmotic or hypertonic solutions cause several different types of injuries to the endothelium. Blood cells exposed to hypertonic saline undergo dehydration and cell wall disruption, but this probably is not the principal mechanism by which endothelial cells are affected. Instead, concentrated osmotic or ionic solutions may partially denature cell surface proteins in situ. Tight junctions between cells are immediately affected, exposing lower cell layers to the hyperosmotic substance.
The time sequence of the remainder of the destructive process is relatively slow, requiring minutes rather than seconds for endothelial destruction.6 For effective sclerosis, the osmotic agent must be of sufficient strength to disrupt the full thickness of the vessel wall for complete destruction.7 Because rapid dilution quickly diminishes the local concentration of the drug, this class of sclerosing solutions is primarily effective for smaller vessels and for low-flow vessels such as leg telangiectasias. There is a smaller amount of inflammation produced by this class of sclerosing agents. A side-by-side comparison will frequently show less hyperpigmentation with the hypertonic solutions (Figure 14-1).
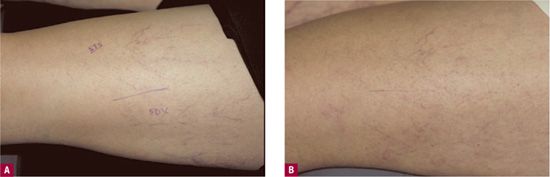
![]() FIGURE 14-1 Comparison of a test site on the thigh of Sclerodex® versus STS with similar concentrations. A. Lateral venous system before treatment with 0.1 percent Sotradecol® (STS) anteriorly and Sclerodex® (SDX) laterally. B. After one month, slightly more hyperpigmentation is present at the STS site and slightly more matting at the SDX site.
FIGURE 14-1 Comparison of a test site on the thigh of Sclerodex® versus STS with similar concentrations. A. Lateral venous system before treatment with 0.1 percent Sotradecol® (STS) anteriorly and Sclerodex® (SDX) laterally. B. After one month, slightly more hyperpigmentation is present at the STS site and slightly more matting at the SDX site.
Hyperosmotic solutions are nonselective in their effects, affecting all cells in the path of the osmotic gradient. Nerve endings in the vessel adventitia can be affected, causing a focal, often intense, burning pain upon injection. Changes in the ion gradient of the extracellular fluid of muscles can trigger severe cramping that can last for minutes at a time. Extravasation can cause extensive tissue necrosis and large ulcers that are slow to heal and can cause significant scarring.
Chemical Irritants or Corrosive Agents
Corrosive agents, the most diverse group of sclerosing solutions, most likely poison cell surface proteins by affecting chemical bonds within the proteins. There is some uncertainty as to which agents really belong to this category. Included are agents such as alcohols, which are not chemical poisons, but are rather solubilizing agents that affect the shape of proteins and the integrity of the cell membrane itself. With further investigation, the category probably will be narrowed to include only chemically reactive compounds that interact with cell surface proteins.
Substances now categorized as toxic or corrosive agents range from alcohols to salts to heavy metals. They act on all cell types, but are consumed in stoichiometric proportions in the process, somewhat limiting the concentration of cell injury remote from the injection site. This category includes some of the strongest solutions, such as polyiodinated iodine (PII), but also includes one of the mildest solutions, such as chromated glycerin (CG). Non-CG compound is now considered to be a hyperosmotic agent. The strongest corrosive solutions may cause full-thickness vessel injury at the site of injection within seconds, facilitating the treatment of large varicosities. It is essential to select the proper volume and concentration so that the destructive effect does not spread beyond the targeted vessel into adjacent tissues.
The most commonly used sclerosing solutions include STS, POL, CG, 72 percent glycerin compound, hypertonic saline, hypertonic saline with dextrose, and iodine. These are widely used throughout the world. For easy reference, the following panels describe each solution.
SODIUM TETRADECYL SULFATE (NONFOAMED SOLUTION) Sodium tetradecyl sulfate (Sotradecol®; AngioDynamics, Inc., Bioniche Teo, Inverin, Co. Galway, Ireland) is a long-chain fatty acid salt with strong detergent properties. It is a very effective sclerosing agent that was already in commercial use before the adoption of The Federal Food, Drug and Cosmetic Act of 1938, and was therefore grandfathered by the FDA without the need to prove safety and efficacy. There are over 50 years of clinical data available, however, on file with manufacturers in the United States and the United Kingdom. The safety and efficacy profile based on hundreds of thousands of treatments is extremely good.
Class Detergent. It may be foamed with agitation and gas such as air.
General Description A reasonably safe, all purpose, fairly strong sclerosant.
Advantages
1. Roughly twice as potent as POL at the same concentration.
2. Can be used in all sizes and types of varicose, reticular, or telangiectatic veins.
3. Intravascular injection is painless.
4. Allergic reactions may occur, but are uncommon (minimized with latex-free syringes).
5. The liquid is fluid, rather than viscous.
6. May be incorporated into a foam solution.
Disadvantages
1. Extravasation may cause necrosis.
2. Hyperpigmentation and telangiectatic matting are not uncommon.
3. May dissolve rubber syringe plunger in higher concentrations.
Principal Indication Large and small varicose veins, reticular veins, and telangiectasias.
Accessory Use Telangiectasias of face (very rarely recommended) and trunk.
Concentrations and Doses in Varicose Veins The maximum concentration is used for treatment at the saphenofemoral junction. When used for truncal or nontruncal varices below the saphenofemoral junction, concentrations of 1.5–3 percent are used for veins greater than 4 mm in diameter and concentrations of 0.5–1.0 percent are used for veins 2–4 mm in diameter.
A volume of 0.5–1.0 cc is normally used at each injection site, and according to the American manufacturer, the total amount per weekly session should not exceed 300 mg (10 cc of 3 percent solution). We agree with the British manufacturer of Fibrovein® (STD Pharmaceuticals, Hereford, UK) form of STS who recommends a lower maximum dose of 120 mg (4 cc of 3 percent solution) per session.
Concentrations and Doses in Telangiectasias and Reticular
Veins Reticular veins are treated with 0.2–0.5 percent solutions and up to 1 cc per site. Telangiectasias are treated with 0.1–0.3 percent solutions and 0.1 or 0.2 cc per site. The recommended starting concentration for telangiectasias is 0.1 percent.
Comments
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


