Fig. 43.1
The da Vinci surgical systems (Courtesy ©2013, Intuitive Surgical, Inc)
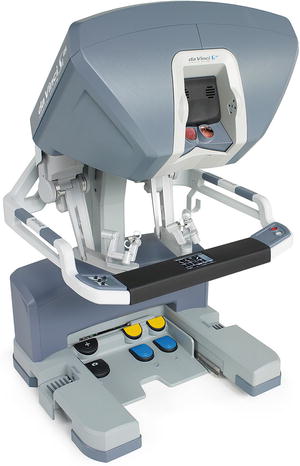
Fig. 43.2
The da Vinci surgical console (Courtesy ©2013, Intuitive Surgical, Inc)
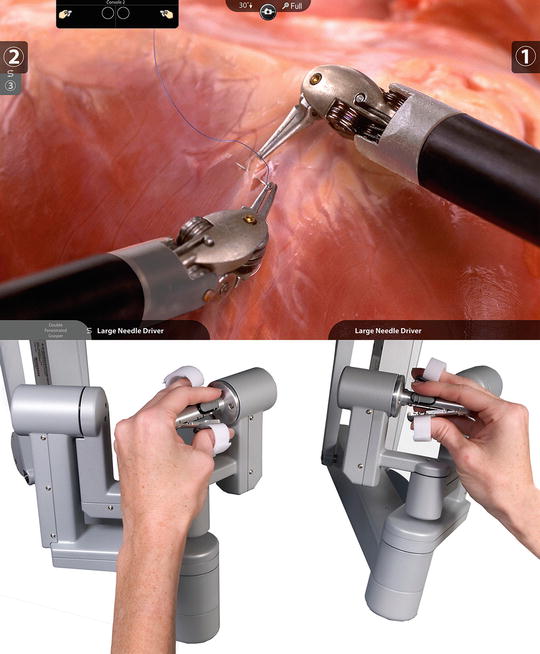
Fig. 43.3
The da Vinci master controllers (Courtesy ©2013, Intuitive Surgical, Inc)
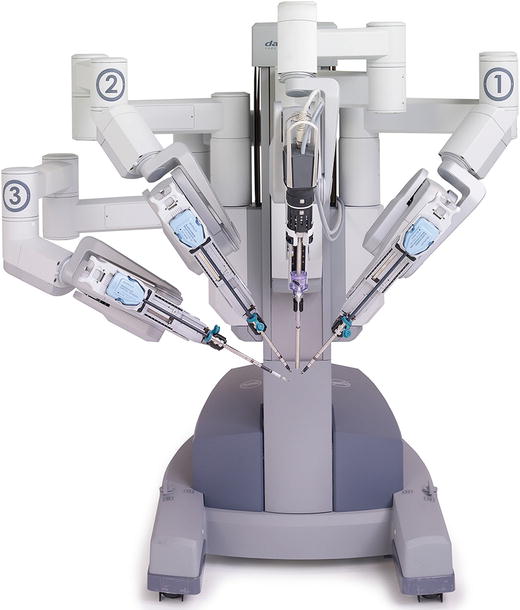
Fig. 43.4
The da Vinci patient cart (Courtesy ©2013, Intuitive Surgical, Inc)
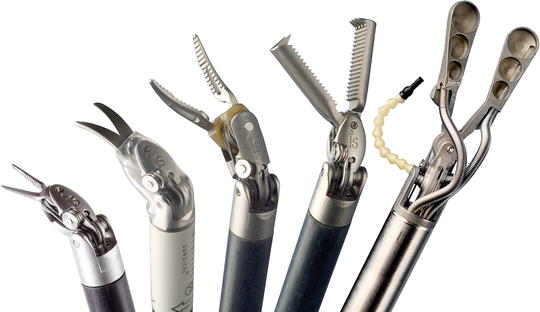
Fig. 43.5
The da Vinci EndoWrist instruments (Courtesy ©2013, Intuitive Surgical, Inc)

Fig. 43.6
The da Vinci vision cart (Courtesy ©2013, Intuitive Surgical, Inc)
Robotics for Bariatric Surgery
Bariatric surgery is rapidly evolving in the field of surgery as the prevalence of obesity continues to rise in epidemic proportion throughout the world. Surgery currently represents the most successful long-term treatment of obesity and its associated comorbidities. However, operating on obese patients presents a particular challenge due to the physical hurdles caused by the excess body mass. While bariatric procedures were originally performed via open surgery, minimally invasive techniques have largely replaced the open approach, and the advantages of a minimally invasive approach have been well validated with reduced postoperative pain, shorter hospital stay, and lower postoperative mortality [1, 2]. Despite its general feasibility, there are a number of technical limitations associated with performing laparoscopic surgery on obese patients, including limited motion of laparoscopic instruments due to a thick abdominal wall, hepatomegaly, and increased amounts of intra-abdominal fat with limited workspace; reduced surgical dexterity; and poorer ergonomics placing significant musculoskeletal stress upon the surgeon. Several publications from the field of gynecology have described clinical benefits of robotic surgery when operating on obese patients [3, 4]. Interestingly, the first robotic procedure was a bariatric procedure, a robotic placement of an adjustable gastric band, performed by Dr. Guy Cadiere and Dr. Jacques Himpens (Belgium) in 1998 [5] using an early version of the da Vinci system prior to its actual market launch. Since then, all of the commonly performed bariatric surgical procedures including Roux-en-Y gastric bypass, adjustable gastric band, sleeve gastrectomy, and biliopancreatic diversion with duodenal switch have been performed robotically and demonstrated to be feasible and safe [6, 7].
Roux-en-y Gastric Bypass (RYGB)
At present, the largest body of literature on application of the robotic platform in bariatric surgery is with the RYGB. Early reports of robotically performed RYBP first appeared in 2003 [8]. Since then, a variety of techniques have been described in a number of publications ranging from a hybrid approach to a totally robotic setup [6, 7]. While some of the early publications highlighted challenges particular to the robotic system such as the absence of tactile feedback leading to bowel injuries from the graspers [9], overall results were encouraging and demonstrated both feasibility and safety with complication rates comparable to those of the laparoscopic procedure [8–15]. Sanchez et al. published the results of the first, and currently the only, prospectively randomized trial comparing robotic versus laparoscopic RYGB [12]. This group randomized the new laparoscopic fellow’s first 50 RYGB patients to either the laparoscopic or robotic approach. The fellow was a novice to both techniques, and thus the results represented the learning curve for both techniques. The investigators found moderately shorter operative times with the robotic approach and also found that the shorter operating time was maximized in patients with a larger body mass index (BMI). A relatively short learning curve for the robotic RYGB of about 10–15 was also concluded by additional research from the same group and another center from Europe [10, 11, 16]. These findings are significantly below the described learning curve of more than 100 cases for the laparoscopic RYGB [17, 18]. Unfortunately, the work by Sanchez and associates remains the only level one study in the field of robotic bariatric surgery. Although there would be equipoise in a randomized clinical trial comparing the robotic versus the laparoscopic technique, most surgeons have gravitated toward one technique versus the other, and it would be challenging to find a surgeon who is equally skilled in both techniques, making it difficult to eliminate the surgeon’s skill bias. Nevertheless, comparative studies [10, 19–26] as well as systematic reviews [6, 7] do offer some tendencies toward robotic bariatric surgery. Most of the studies involving comparisons between the laparoscopic versus robotic approach to the RYGB suggest that the robotic approach is associated with equal or lower complication rates. However, studies find that the robotic operative times tend to be longer than for the laparoscopic approach [9, 13, 21, 23–26], although there are studies that have found shorter operative times with the robotic approach [10, 12, 19, 22]. The largest reported series, 1,100 patients, by Wilson et al. clearly establishes the safety of the robotic RYGB and compares favorably with any large reported laparoscopic series [27].
The size of stomach pouch, length of biliary and alimentary limbs, and the method of formation of the gastrointestinal and intestine-intestinal anastomoses are not strictly standardized. These elements of the gastric bypass, particularly the creation of the gastrojejunal anastomosis, have been influenced by the transition from open to laparoscopic approach. The technical challenges of creating a sutured anastomosis laparoscopically caused the shift by many surgeons to a stapled technique. A fundamental driver for the introduction of robotics to this procedure was the belief that a hand-sutured gastrojejunal anastomosis would improve the quality of the anastomosis when compared to the stapled technique and that hand suturing would be greatly facilitated by the enhanced dexterity of the robotic system. Although different techniques have been described for the formation of this anastomosis similar to the laparoscopic approach, there is a predominance of a double-layered completely sutured technique among surgeons who have adopted the robotic technique. Comparative data suggest a lower leak and stricture rate of the gastrojejunal anastomosis when using a robotically sutured technique compared to laparoscopic stapling [21–23, 25]. The advantage in terms of postoperative strictures was further recognized in a meta-analysis by Markar et al. [7]. Finally, some evidence exists that there may not be a difference in short-term weight loss between the robotic versus the laparoscopic approach to the RYGB [6, 7].
Sleeve Gastrectomy (SG)
Most of the reported literature on robot-assisted sleeve gastrectomy (RASG) supports the feasibility and safety of the robotic approach, but generally find longer operative times for the procedure [28–31]. Ayloo et al. [28] compared robotic gastric sleeve resection to the laparoscopic procedure with similar clinical outcomes. The learning curve with the RASG has been estimated to be completed after about 20 cases [30].
Unlike the biliopancreatic diversion with duodenal switch (BPD/DS) and the RYGB, the advantages of the robotic approach to the SG over the laparoscopic approach have historically been less clear. The robotic approach can facilitate exposure during the mobilization of the stomach, as well as facilitate the oversewing of the long gastric staple line. However, this is mitigated by the fact that the stapling portion of the procedure, which is arguably the most critical element of the procedure, is performed by the bedside assistant, whether this is another MD or a PA. Additionally, the most commonly used energy sources for dividing vessels in robotic SG, the harmonic scalpel or LigaSure™, are non-wristed instruments. For this reason, the earliest reported studies [28] involved essentially a completely laparoscopic approach, with the robot being used to simply oversew the gastric staple line. Recently, with the release of the EndoWrist® vessel sealer, which provides a wristed energy source to dissect the vessels from the greater curvature of the stomach and, more importantly, the recent Food and Drug Administration (FDA) approval of the robotic stapler, the surgeon can perform all of the elements of the SG from the console and benefit from the inherent advantages of the wristed instruments as well as the ergonomic advantages of the system.
Adjustable Gastric Band (AGB)
The first robotic bariatric procedure performed was a robotic placement of an AGB by Cadiere and Himpens in 1998 [5]. Subsequently other authors have reported on the feasibility and safety of performing a robotic AGB [31, 32]. Although the wristed instruments provide some advantages for creating the posterior gastric tunnel and suturing the anterior gastric plication, the lack of tactile feedback can be a significant disadvantage in the “blind” passage of an instrument to create the posterior gastric tunnel. More importantly, the AGB is technically a low complexity case with very few perioperative complications when performed laparoscopically, and most surgeons agree that there is very little advantage or role of the robotic approach to the AGB. Muhlman et al. [31] compared four robotic gastric bands to four laparoscopic procedures. While there was one intraoperative band removal that was necessary due to a suspected injury of the stomach during one of the robotic procedures, no clear statement could be concluded from this publication due to the small study size. Edelson et al. [32] showed comparative perioperative morbidity of robotic and laparoscopic gastric banding in their overall patient population; however, in patients with a preoperative BMI ≥50 kg/m2, the operating time was shorter using the robotic approach.
Biliopancreatic Diversion with Duodenal Switch (BPD/DS)
The BPD/DS, because of the technical complexity of the operation, is the only bariatric surgery that is still performed openly more than laparoscopically. The duodenal dissection, as well as the duodeno-ileal anastomosis, is particularly challenging. The wristed instruments, greater maneuverability and precision of dissection, enhanced visualization, and ability to suture all greatly facilitate these two components of the procedure, and thus, there is an obvious appeal for the use of the robot. Despite the benefits of the robotic system, the complexity inherent into the procedure is reflected in Sudan et al.’s [33] retrospective review of 120 consecutive patients undergoing robotic-assisted laparoscopic biliopancreatic diversion with duodenal switch (RA-LBPD/DS). Although there was no mortality, 18.3 % of the patients either experienced high blood loss (13.3 %), conversion (2.2 %), or leaks (5.8 %). The investigators, however, found that the complications were strongly predicted by increasing surgeon experience and declined significantly after 50 cases. Publications on the use of the robotic platform in BPD/DS are, otherwise, very limited. A technical limitation to its use is that the BPD/DS requires access to almost the entire abdominal cavity from the gastroesophageal junction during the performance of the sleeve gastrectomy component to the right lower quadrant for measuring the small bowel from the ileocecal valve, as well as for performing an appendectomy, which is commonly done as part of the procedure. This would require multiple dockings to optimally position the robotic arms to perform the sleeve gastrectomy, the duodenal dissection and anastomosis, and the lower intestinal measurement and anastomosis. A more likely reason for the limited use and scarce reporting of the use of the robot in BPD/DS is that this is not a commonly performed procedure and represents less that 5 % of the bariatric procedures performed in the USA; therefore, there is less likely to be overlap among surgeons who perform the BPD/DS, surgeons who have access to a robotic system, and surgeons who are early adopters of this technology.
Revisional Surgery
The complex nature of revisional bariatric surgery makes it potentially ideal for the robotic approach. The superior visualization and enhanced dexterity allow a safer dissection within tissue planes and facilitate the identification of critical structures. Additionally, the ability to perform precise intracorporeal suturing is critical in reconstructing the anatomy, which often involves reconstruction of the gastrojejunal anastomosis in settings that compromise the ability to use a stapler for the anastomosis. The published literature on the use of the robot in revisional surgery is limited at present to a single retrospective study on prospectively collected data by Snyder et al. [25] that included 99 patients who underwent robotic-assisted revisional procedures for either failure of weight loss or weight regain, complications or intolerance of the primary surgery, or for other health concerns between 2004 and 2011. Two-thirds of the procedures (65/99) were revisions of an adjustable gastric band to either SG or RYGB. In all instances that a gastrojejunal anastomosis was required, this was performed as a sewn anastomosis using the robot. Among the study findings were a 0 % leak rate and a 0 % mortality rate. Although their study population differed from that of Rosenthal et al. [34




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






