22 Repair and grafting of peripheral nerve
Synopsis
 Primary neurorrhaphy is the gold standard by which all other nerve repair techniques are judged.
Primary neurorrhaphy is the gold standard by which all other nerve repair techniques are judged.
 Primary repair, delayed primary repair, and secondary repair indicate timing of the repair with respect to the injury.
Primary repair, delayed primary repair, and secondary repair indicate timing of the repair with respect to the injury.
 Excessive tension will inhibit nerve regeneration; however a small amount of tension to achieve primary coaptation is acceptable.
Excessive tension will inhibit nerve regeneration; however a small amount of tension to achieve primary coaptation is acceptable.
 Nerve autograft is the gold standard for reconstructing a nerve gap.
Nerve autograft is the gold standard for reconstructing a nerve gap.
 In the event of a nerve gap, options for repair include:
In the event of a nerve gap, options for repair include:
 Factors most affecting nerve recovery include:
Factors most affecting nerve recovery include:
Types of nerve injury
Nerve injury
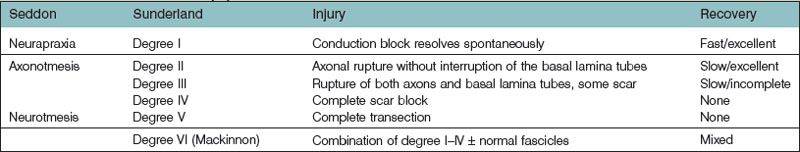
A peripheral nerve injury can be classified in several ways. Historically, the first classification system by Sir Herbert Seddon (1943) was based on gross and histologic anatomical changes rather than mechanism of injury.1 He described three types of nerve injuries. Firstly, neurapraxia involves a local conduction block at a discrete area along the course of the nerve. Wallerian degeneration does not occur and the recovery is excellent. Secondly, axonotmesis implies direct axonal damage, while thirdly, neurotmesis is the transection of a peripheral nerve. In both axonetmesis and neurotmesis, wallerian degeneration occurs distal to the site of injury; however while the former recovers, the latter does not. Within axonotemesis, Seddon described a degree of injury associated with scarring and less complete recovery. Within neurotmesis he described an “in continuity,” total scar, and no recovery injury. However it was Sunderland who expanded upon the earlier Seddon classification and emphasized five degrees of nerve injury.2 Mackinnon later went on to include a sixth (Table 22.1).3,4 First-degree (neurapraxia) and second-degree (axonotmesis) injuries recover spontaneously, the latter at the classic rate of 1 inch (2.5 cm)/month or 1–1.5 mm/day.5 Fourth- and fifth-degree injuries do not recover, while third- and sixth-degree injuries recover partially for different reasons. The difficulty with surgical correction of a sixth-degree injury is limiting the repair to the fascicles affected by fourth- and fifth-degree damage and not damaging fascicles with the potential for spontaneous recovery.
Nerve injuries can also be grouped by mechanism of injury and whether they are open or closed. This clinical classification is useful in evaluating nerve injuries, determining the likelihood for spontaneous recovery, and provides an algorithm for managing these injuries. Table 22.2 lists the typical nerve injury patterns and their significance with respect to assessment and treatment. For simplicity, nerve injuries can be grouped as: (1) penetrating injuries; (2) crush and compression injuries; and (3) stretch and avulsion injuries. While generally, avulsion and crush injuries tend to be closed injuries, they may be open. This implies a more severe force, is associated with soft-tissue, vascular, and orthopedic injuries and the extent of muscle and skin damage will ultimately influence the outcome of the nerve repair. More extensive soft-tissue and/or bony injuries raise the complexity as well as the likelihood for a staged reconstruction. Often, the nerve reconstruction is delayed in light of more acute vascular and orthopedic injuries.
Table 22.2 Classification of nerve injury by mechanism. The significance of each type of injury in terms of ability to repair and outcome is discussed
| Type | May be injured | Significance |
|---|---|---|
| Stretch/avulsion injury | ||
| Avulsion | Nerve roots | Unable to be repaired primarily |
| Nerves exiting | Indication for nerve transfer | |
| Foramen, bony fracture | ||
| Stretch | Any nerve | Mixed nerve injury (degree VI) |
| Crush and compression injury | ||
| Injury | ||
| Complex crush | Skin, subcutaneous | Varying degree of depth, loss of function is related to amount of tissue destruction |
| Tissue, muscle, nerve, ± bone | ||
| Chronic compression | Nerve | Slow onset, reversible |
| Acute compression | Nerve ± muscle | Quick onset, reversible muscle ischemia, variable recovery of both muscle and nerve |
| Compartment syndrome | Nerve + muscle | Quick onset, reversible muscle ischemia if ischemia less than 6 hours; no or variable recovery of muscle if released after 6 hours |
| Penetrating injury | ||
| Sharp | Skin, subcutaneous tissue, muscle, nerve ± bone | Needs surgical exploration because of high probability of nerve severance |
| All levels | ||
| Blunt | Variable | Injury may extend further than expected |
| Blast | Variable | Injury pattern depends on ballistic makeup and velocity |
| Electrical | Variable | Neuropathy is from damage to myelin sheath and ranges from neuropathy to causalgia |
Penetrating injuries
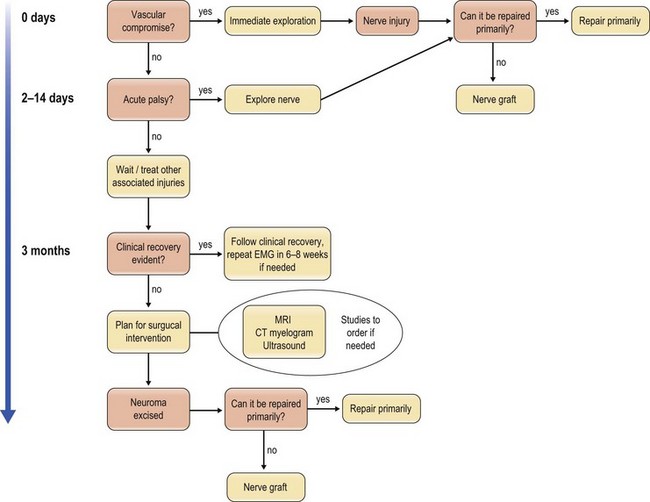
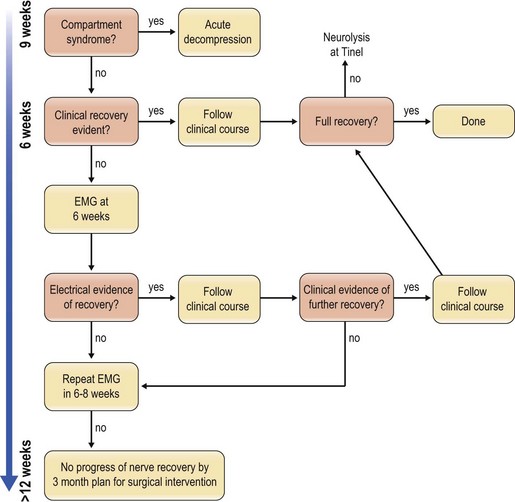
Blunt penetrating and blast injuries are usually treated conservatively, similar to closed crush and stretch injuries, because they are may recover spontaneously. The local-tissue edema often causes a neurapraxia that resolves; however, those that do not recover after 3 months should be evaluated by electrodiagnostic studies and the algorithm for traction or crush injury followed (Fig. 22.1).
Crush injuries
Crush injuries comprise the most common peripheral nerve injuries to the extremity. External compression may be complicated by increased internal pressure from hematomas, fractures, and local tissue edema. When minor, this may cause a temporary neurapraxia, but with greater compression, the likelihood of a permanent injury increases. The most severe consequence of a crush injury is the progression to compartment syndrome, which is a surgical emergency. Often an early sign of impending compartment syndrome is a decrease in vibration sensibility.6 Compartment syndrome is discussed in greater detail in another chapter.
The nerve portion of the injury is usually treated conservatively and exploration is warranted if the nerve recovery does not follow an expected pattern. Along with avulsion injuries, the algorithm is to follow recovery for 3–4 months and intervene if the expected recovery does not appear to be forthcoming (Fig. 22.2).
Stretch and nerve avulsion injuries
Nerves that are stretched to the point of avulsing from the proximal end suggest a high-velocity or high-impact injury that is often associated with limb-threatening injuries that take precedence over the nerve injury. The nerves tend to be avulsed around areas of tethering, such as bony foramina and the spinal cord. If the proximal nerve can be accessed, as in injuries of the obturator nerve in the pelvis, a graft can be placed through the orifice and a neurorrhaphy with nerve graft may be performed. For areas such as the cranial nerves or the spinal roots, where the portion proximal to the avulsion is either central nervous tissue or inaccessible due to skeletal constraints, reconstruction is done via nerve transfers.7 Our current surgical algorithm focuses on nerve reconstruction first, followed by tendon transfers and associated procedures to augment or complement the nerve reconstruction when warranted (Fig. 22.2).8
Nerves avulsed at the neuromuscular junction present a different problem, as the distal end is unavailable. In the case of motor nerves that are avulsed from the muscle bellies, implantation of the proximal nerve directly into the muscle is the alternative when no distal end is available. Some studies show as good as M4 motor recovery 1–2 years after direct nerve to muscle neurotization9; however, experimental studies do not support these findings; rather, recovery is much less than a nerve coaptation would produce.10
Evaluation of nerve injuries
A thorough physical exam remains the most reliable method for determining the neurologic defect. Sensation, or lack thereof, can be tested using Semmes–Weinstein filaments, two-point static and moving discrimination, or the “ten test.” The quick and easy ten test sensory exam uses the patient’s own subjective perception to moving light touch in order to elicit differences in sensation.11 For example, to test for a median nerve injury, both the injured and uninjured index fingers of the patient are touched at the same time over corresponding areas of each finger and the patient is asked if the subjective sensation is the same or different. This technique is particularly useful in young children who may not be able to cooperate with a two-point discrimination test.
Nerve repair
Tension and nerve repair
Excessive tension across a nerve repair is known to increase the scarring at the coaptation site and impair regeneration. In an uninjured nerve, a 15% strain of the nerve causes a reduction in microvascular flow and, for an hour after relaxation, a delay of peak velocity to 66% of initial value persists.12 Mild tension, on the other hand, is actually believed to be beneficial to the repair by stimulating neurotropic growth factors.
In clinical practice we do not formally measure the tension across a neurorrhaphy; however we always avoid tension on a repair. The two ends of a nerve are mobilized in order to bring the ends in reasonable approximation. Strain on the nerve decreases the microcirculation and excessive tension will cause the repair to break down. In addition, tricks such as positioning the arm in adduction or placing the wrist and fingers in flexion will significantly decrease the tension across the repair depending on the location of the injury and may increase the total length of available nerve; however, we recommend avoiding postural manipulation in order to force primary repair. Not only can dehiscence occur when the joint near the repair is moved too early, but it may create significant stiffness from prolonged immobilization.13 An immobilized nerve also results in scarring at the repair site.
Type of repair: epineural versus fascicular
Any repair that withstands gentle range of motion is considered a “tension-free” repair. Giddins et al.14 showed in a cadaveric study comparing suture size to the strength of an acute repair of the median nerve that 9–0 nylon withstood the greatest distractive forces. At a lower tension 10–0 snapped, and 8–0 sutures pulled out of the nerve tissue. However, in clinical practice, 10–0 and 8–0 sutures are often used based on the size of the nerve, the thickness of the epineurium, and the amount of inflammation.
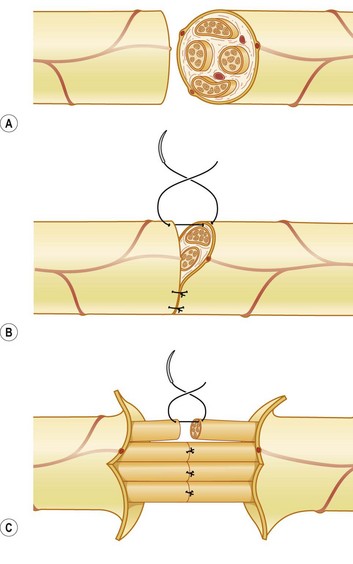
Epineural repair is the preferred method of repair once the severed ends are freshened surgically. External markers such as a vessel on the surface and matching the fascicular patterns are used to align the fascicles without overlapping (Fig. 22.3). For major peripheral nerves, an argument for perineural repair can be made to improve alignment of the larger fascicular groups individually; however, clinical studies support both techniques as equally effective as long as the fascicles were not overlapped (Fig. 22.4).15 The disadvantage of a perineural repair is that the extensive dissection and the permanent intraneural stitches can lead to increase fibrosis.16 In practice, it is often difficult to align the fascicles accurately, as trauma, edema, and scarring can distort the normal topography.