Reconstruction of the Scalp
Anatomy
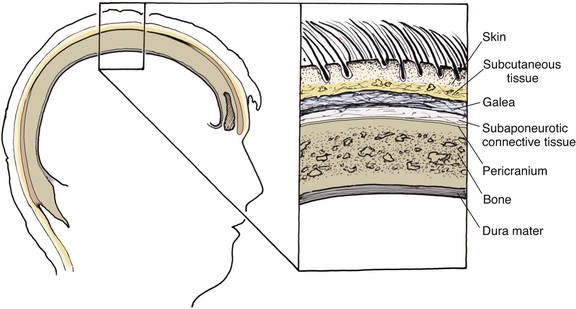
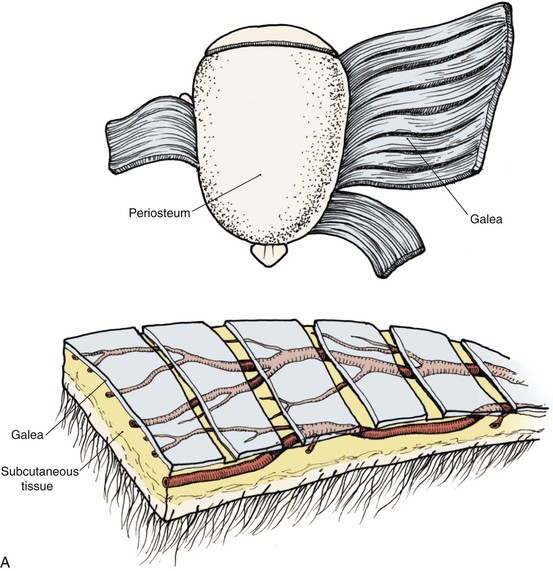
The anatomic layers of the scalp are well described by the convenient acronym SCALP (Fig. 24-1): S, the skin; C, the subcutaneous tissue; A, the galea aponeurosis; L, the loose connective tissue; and P, the periosteum, also known as the pericranium. The scalp has the thickest skin on the body. However, the thickness of scalp skin not only varies across the surface of the head but also may change throughout one’s life. The scalp skin is thickest when hair growth is at its most robust. If the hair thins, as in male pattern baldness or pathologic alopecia, scalp thickness significantly decreases. The subcutaneous fat layer contains hair follicles and integumentary structures such as sweat and sebaceous glands. It provides a tight connection between the skin and the underlying galea. The galea is a dense, inelastic layer that provides a fibrous connection between the frontalis muscle anteriorly and the occipitalis muscle in the nape of the neck. Within and adjacent to the galea run numerous vascular and neural structures. In the forehead region, the galea envelops the frontalis muscle, which has no direct attachment to bone. Posteriorly, the galea encompasses the occipitalis, which inserts in the bone along the nuchal line. The periosteum is usually tightly affixed to the cranium and possesses its own vascular supply.
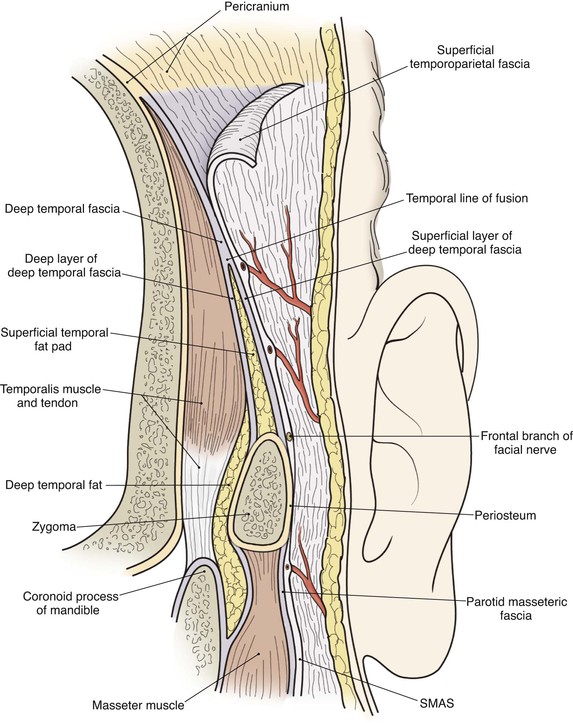
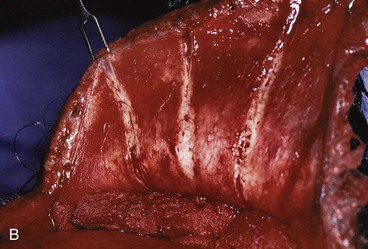
In the region of the temple, the anatomic layers of the scalp are more complex. Medial to the temporal line, which represents the superior aspect of the temporalis muscle, the SCALP acronym applies. However, over the temporalis muscle, the layers of the scalp differ slightly from the remaining scalp (Fig. 24-2). Over the superior aspect of the temporalis muscle, the first three layers of the scalp, the skin, subcutaneous fat, and galea, remain the same. Inferiorly, however, the galeal layer of the temporal region becomes the temporoparietal fascia. The temporoparietal fascia is loosely attached to the subcutaneous fat and is continuous with the fascia of the frontalis muscle in the forehead region. The temporoparietal fascia accounts for the difference between the loose and tight attachment of the scalp to the cranium (Fig. 24-3). The tight region of the scalp has a dense galeal layer, no underlying muscle, and poor distensibility. In the loose regions of the scalp, the galea is thinner and overlies muscle anteriorly as well as posteriorly below the nuchal line and is considerably more distensible. It is important to understand this anatomic difference to be successful in planning scalp flaps. For example, small advancement and transposition flaps are effective for repair of medium-sized (3 cm) defects in the loose regions of the scalp. Such flaps are not as effective for repair of similar-sized defects in the tight regions of the scalp because of the inelastic nature of the tissues in these areas.
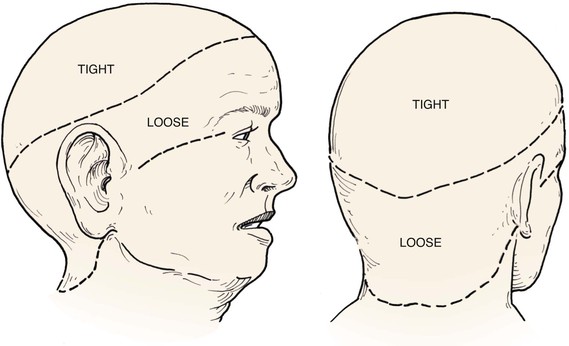
FIGURE 24-3 A, B, Tight and loose regions of scalp. Tight region has dense galeal layer, no underlying muscle, and poor distensibility. In loose region, the galea is the thin fascial layer overlying muscle, and scalp is more distensible.
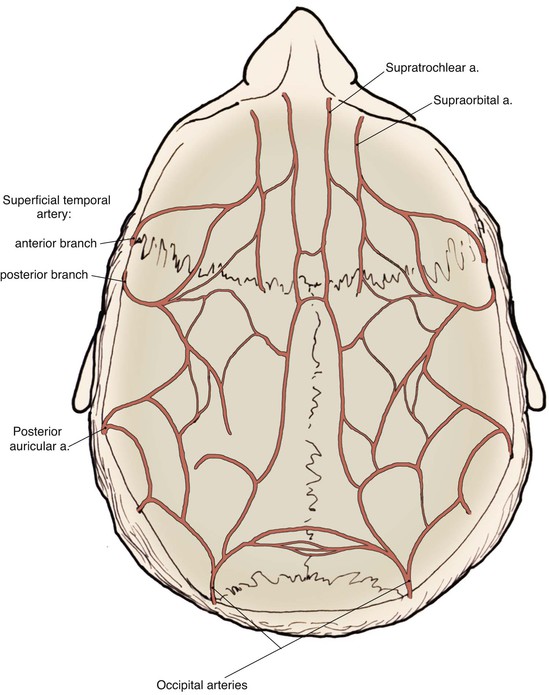
The scalp has a rich and redundant vascular supply (Fig. 24-4). The external carotid artery provides the majority of the blood supply to the scalp through the superficial temporal, posterior auricular, and occipital arteries. The internal carotid system also supplies the scalp anteriorly through its terminal supraorbital and supratrochlear arteries, which course up the forehead after exiting the orbit. The robust vascular network of the scalp will usually support large random patterned local flaps with variable configurations. However, there are limited anastomotic connections across the midline. Thus, flaps that extend a considerable distance across the midline may benefit from delay. This is particularly true in children.1 When designing flaps, the surgeon should be cognizant of the location and typical course of the principal arterial branches that supply the scalp and try to incorporate them in the base of the flap. A Doppler probe may be helpful in outlining the course of these vessels through the thick overlying skin. Many of the larger vascular branches run within the galea and temporoparietal fascia. For preservation of these vessels, scalp flaps are ideally dissected just deep to this layer within the loose connective tissue, creating a fasciocutaneous flap. The galea provides some protection to the vascularity of scalp flaps by limiting the elongation and crimping of vessels. The skin and subcutaneous tissue layers of the scalp also have a dense vascular plexus, but there is a greater risk of ischemia when flaps are dissected in the subcutaneous tissue plane. In addition, elevating a flap in the subcutaneous tissue plane subjects the hair follicles to the risk of injury and subsequent hair loss. The principal disadvantage of flaps that include the galeal layer is that the flaps are inelastic and nondistensible. Hence, scalp flaps must be designed with a large surface area relative to the size of a defect the flap is intended to repair. In addition, wound closure tension is likely to be higher compared with local cutaneous flaps used in other areas of the head and face.
The layers of the scalp provide convenient surgical dissection planes. Dissection beneath the galeal layer is simple, swift, and relatively avascular. Large flaps can be readily dissected in this tissue plane with minimal effort and good reliability. Flaps dissected in the subcutaneous plane compared with subgaleal dissection are considerably more difficult as this is a “nonanatomic” plane that requires sharp surgical dissection. Typically, these types of flaps are elevated just below the hair follicles within the subcutaneous fat. Numerous small vessels are encountered and hemostasis can be difficult. In addition, electrocautery used to secure hemostasis can injure the hair follicles. For these reasons, scalp flaps consisting only of skin and subcutaneous fat are rarely employed. In the area over the temporalis muscle, the temporoparietal fascia can be raised as an independent flap on a pedicle or as a microsurgical free flap and transferred to an adjacent or distant site.2,3 Incisions for the flap must remain posterior to the course of the temporal branch of the facial nerve to avoid denervation of the frontalis and corrugator muscles. The temporoparietal fascia flap is useful in auricular and orbital reconstruction as it provides thin, supple, and highly vascular tissue supplied by the posterior branch of the superficial temporal artery.4,5 Details of this flap are discussed elsewhere in this chapter. Flaps consisting of periosteum can also be developed independent of the overlying scalp tissues.
Healing by Secondary Intention
In some instances, allowing a scalp defect to heal by secondary intention may be a good option.6 However, several criteria should be met before this option is selected for the treatment of defects of the scalp. Most important, there must be a base of viable tissue, such as pericranium, on which granulation tissue can form. On occasion, bare bone may support the formation of granulation tissue on its own, but the process is exceedingly slow and fraught with problems. The surgeon can enhance the formation of granulation tissue by removing the outer table of the cranium to the level of the diploic space, which is quite vascular. This may allow islands of granulation to form, which will then coalesce into a continuous layer that can support re-epithelialization of the wound or a skin graft.
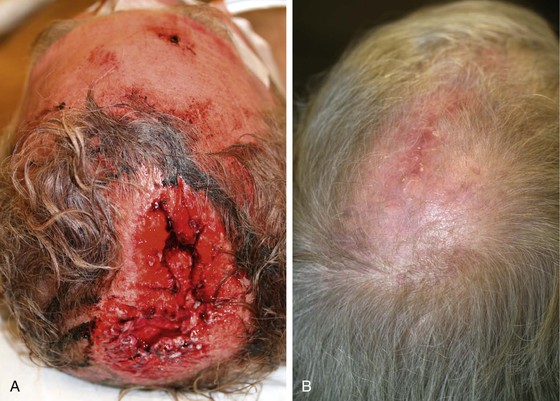
There are several drawbacks to allowing scalp defects to heal by secondary intention. Unlike wounds elsewhere on the head and neck, scalp wounds will minimally contract because of the stiff galea. Thus, it may require many weeks for a wound to heal. Wound care can be laborious and tedious for the patient or the caregiver. When healing has occurred, the area will be devoid of hair, and the regenerated skin covering the area is significantly thinner than the skin of the surrounding scalp. The thin skin has a propensity to break down into an open wound from minimal trauma to the area. In areas that have previously been irradiated or otherwise traumatized, the healing may be incomplete or extremely slow. Nonetheless, in selected patients and in certain areas such as the vertex in a bald patient, the result of wound healing by secondary intention can be acceptable (Fig. 24-5).
Primary Wound Closure
In general, defects of the scalp up to 3 cm in diameter may be repaired with primary wound closure. However, to accomplish this, wide undermining of the scalp in the subgaleal plane is required. This can often be accomplished by blunt finger dissection. Galeal relaxing incisions may also be judiciously employed on the undersurface of the scalp to allow some stretching of the galea, thereby reducing wound closure tension (Fig. 24-6). This technique can also be used in transferring scalp flaps. Galeal relaxing incisions must be performed cautiously to avoid damaging the vascular structures that are immediately superficial to the galea. Some authors have advocated intraoperative tissue expansion to assist flap elevation and to facilitate closure of scalp wounds.7,8 This technique is discussed in more detail later in this chapter. Other tissue-stretching devices have been employed as an adjunct to facilitate primary wound repair.9–12 Once the scalp is sufficiently undermined, the wound edges are advanced and apposed in layered fashion. The galeal layer should bear the majority of the closure tension to minimize the tension on the skin closure line. Wound closure tension placed on the galea and not the skin of the scalp avoids trauma to hair follicles and reduces the risk of postoperative alopecia. It is common for standing cutaneous deformities to form at the borders of the defect because of the inelastic nature of scalp. However, most of these will gradually resolve over time and rarely require secondary revision.
Skin Grafting
Split-thickness skin grafts provide a reliable and simple but suboptimal reconstruction of the scalp.13,14 For the majority of patients, a skin graft is distinctly inferior to a flap repair. Although an intact pericranium will readily support a skin graft, this option has numerous disadvantages. One obvious disadvantage is that skin grafts lack hair follicles. In addition, skin grafts are considerably thinner than normal scalp and as a result are associated with contour deformities. Skin grafts also tend to be unsightly, painful, and susceptible to trauma. Skin grafts may be so fragile that the patient cannot wear a hairpiece over it without pain or injury to the graft (Fig. 24-7).
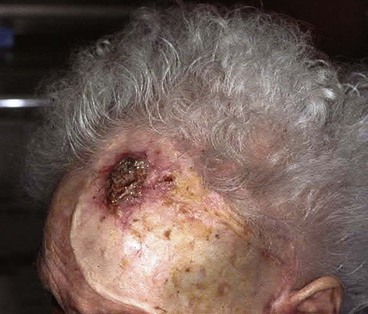
FIGURE 24-7 Repair of large scalp and forehead defect with split-thickness skin graft. Note crusting and ulceration in superior aspect of graft.
In patients who are not appropriate candidates for more extensive procedures and in whom the aesthetic outcome is of less concern, a skin graft may be a reasonable reconstructive option. Skin grafts may be useful to provide temporary immediate coverage of a scalp defect while the patient is being prepared for a more definitive repair with an expanded local flap or microsurgical free flap. Skin grafts may be useful in assisting with the closure of secondary defects, which are frequently located in a less visible area of the scalp, such as the occiput. A skin graft may be indicated when patients have a high risk of local recurrence after removal of a scalp malignant neoplasm. In such instances, repair with a scalp flap is performed at some time in the future after the major risk of recurrence has passed. In most cases, the subsequent removal of the skin graft is accomplished by serial excisions or a single excision followed by a scalp flap with or without prior tissue expansion. Ideally, skin grafts should be placed over viable pericranium, muscle, or fascia. When a sizable area of exposed bone without periosteum is present, an adjacent pericranial, temporoparietal fascial, or muscle flap can be used to cover the exposed bone and serve as a vascularized recipient site for the skin graft. Some skin grafts may even survive over denuded cranium, although certainly this is considerably less reliable and more prone to subsequent traumatic ulceration even when the graft is successful. As with secondary intention healing, there may be some benefit to removing the outer cranial table to allow contact of the skin graft with the diploic space and its vasculature or to allow the wound to granulate before the graft is placed. The best locations for use of a skin graft to repair defects of the scalp are on the vertex in a balding patient and on the forehead. Use of skin grafts should be limited in patients who require postoperative radiation therapy because of the propensity of the graft to break down during or after radiation treatments.
Local Flaps
Scalp flaps can be designed as advancement, pivotal, or interpolated.9 Unlike in other regions of the face, there are no relaxed skin tension lines in the scalp. Thus, incisions can be positioned to maximize vascular supply and tissue recruitment. The principal anatomic landmark that the surgeon should be cognizant of during flap design is the anterior hairline. All incisions in the scalp should be made parallel to the direction of hair growth to minimize hair follicle injury. Electrocautery should be used judiciously for the same reason.
Advancement flaps have limited usefulness for reconstruction of the scalp because of the inelasticity of scalp tissue. Primary closure of a linear wound is in essence two large opposing advancement flaps. Primary wound closure can typically be used to repair small (3 cm or less) scalp defects (Fig. 24-8). The most useful flap employed for the repair of the majority of scalp defects is a rotation flap15–18 (Figs. 24-9 and 24-10). The curvilinear incision necessary to create a rotation flap is well suited to the spherical shape of the cranium. Even relatively small defects require a rather long flap incision. Rotation scalp flaps are designed four to six times as long as the defect is wide. The incision is beveled to parallel the direction of growth of the hair follicles to minimize alopecia along the incision. For defects located in the anterior scalp, the incision is designed to follow the hairline whenever possible (Fig. 24-11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree