(1)
Department of Orthopaedic Surgery, University of Michigan Hospital and Health Systems, Ann Arbor, MI, USA
Abstract
Immediately following its description in 1978, the orthotopic hind limb transplantation in rats became the most commonly used animal model to study acute and chronic rejection in vascularized composite tissue allografts. Although the general principles of hind limb transplantation did not change over time, various authors have modified the original technique in attempt to reduce complication rates (including nonunion, malunion, intraoperative bleeding, postoperative infection), and shorten the operative time. Individual components of the hind limb were also used alone or in combination to test the host’s immune response to various components of the VCA.
Keywords
Vascularized composite allotransplantation (Vca)Limb allograftVascularized bone marrow transplantationVascularized skin allograft face allograftImmediately following its description in 1978, the orthotopic hind limb transplantation in rats became the most commonly used animal model to study acute and chronic rejection in vascularized composite tissue allografts [1, 2]. Although the general principles of hind limb transplantation did not change over time, various authors have modified the original technique in attempt to reduce complication rates (including nonunion, malunion, intraoperative bleeding, postoperative infection), and shorten the operative time [3–6]. Individual components of the hind limb were also used alone or in combination to test the host’s immune response to various components of the VCA [7]. We further modified the model by including the cremaster muscle on the same vascular pedicle with the rat hind limb. This allowed us to use the cremaster muscle as a window to observe microcirculatory changes during allograft rejection [8–10]. This model greatly contributed to our current understanding of VCA rejection. In this chapter, you will find technical details and some preliminary results using this technique.
Surgical Technique
Preparation of the donor: Following induction of anesthesia and preparation of the skin, we made a ventral skin incision over the scrotum extending parallel towards the inguinal ligament (Fig. 20.1a). Thorough this incision, skin and subcutaneous tissues were dissected. A fixation suture using 6.0 silk was applied to the lower end of the cremaster muscle (Fig. 20.1b). We then made a 1 cm incision on anterior abdominal wall (Fig. 20.1c) and removed scrotal contents (Fig. 20.1d). The cremaster muscle was isolated on its vascular pedicle as a tube flap (Fig. 20.1e). We created a pocket at the anterior aspect of the thigh to preserve the cremaster muscle for observation of microcirculation (Fig. 20.1e–g).
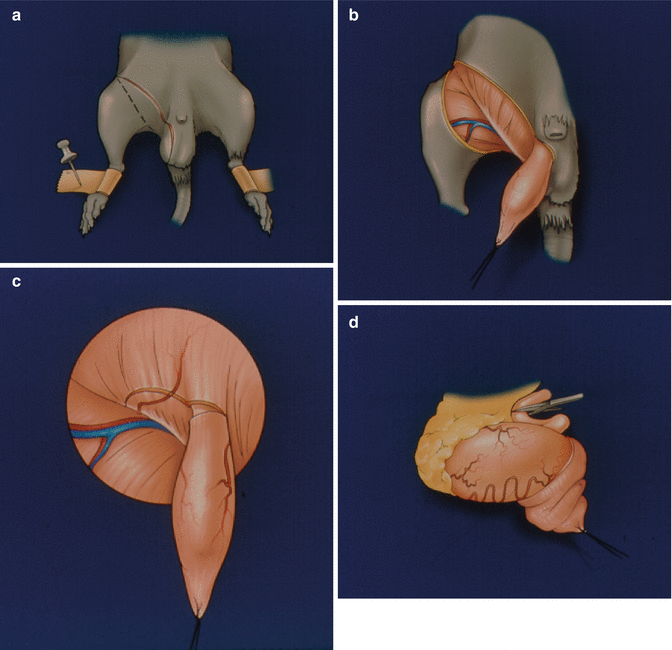
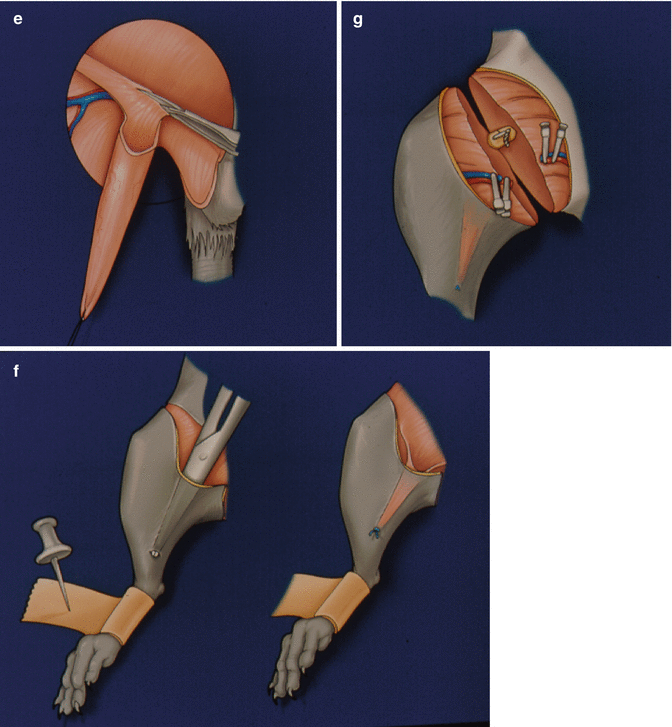


Fig. 20.1
A ventral skin incision over the scrotum extending parallel towards the inguinal ligament (a). Skin and subcutaneous tissues, and a fixation suture on the lower end of the cremaster muscle (b). A 1 cm incision on anterior abdominal wall (c). Removal of scrotal contents (d). The isolated cremaster muscle on its vascular pedicle as a tube flap (e). A pocket created at the anterior aspect of the thigh to preserve the cremaster muscle for observation of microcirculation (f, g)
We then dissected out femoral artery and vein, and followed them above the inguinal ligament. Unlike conventional rat hind limb allograft transplantation model, this model requires such dissection to include the vascular pedicle to the cremaster muscle (Fig. 20.2). Once all side branches were tied, the hind limb was amputated at the midfemoral level.
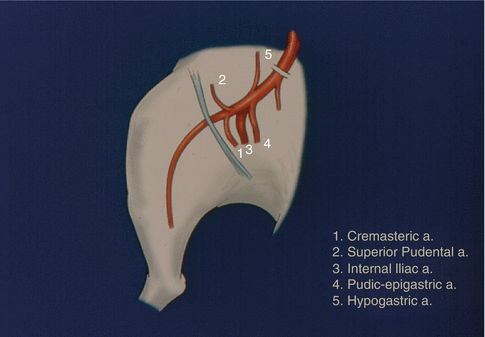

Fig. 20.2
The pedicle of the allograft and the other arteries
Preparation of the Recipient
We made the same ventral inguinal incision on the ipsilateral himb limb. This incision was not extended to the scrotum as the cremaster muscle was not to be removed from the recipient. Following elevation of the fat pad, femoral vessels were indentified. We tied all side branches and amputated the limb at mid-femoral level leaving a longer vascular pedicle on the recipient side.
Transplantation Procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








