Radiation and Radiation Injuries
James Knoetgen III
Salvatore C. Lettieri
INTRODUCTION
Roentgen’s discovery of X-rays in 1895 was closely followed by the introduction of radiation therapy for the treatment of a variety of cancers and other disease processes. Radiation provides both diagnostic and therapeutic benefits, but the resulting changes to exposed tissues pose wound healing problems and reconstructive dilemmas for which the plastic surgeon is often consulted. This chapter explains the basics of radiation therapy, discusses the radiation wound issues that are frequently faced by plastic surgeons, and emphasizes the unique problems posed by specific anatomic locations.
Radiation refers to the high-energy particles (alpha particles, beta particles, and neutrons) and electromagnetic waves (X-rays and gamma rays) that are emitted by radioactive substances (uranium, radon, etc.). Alpha particles are large, positively charged, helium nuclei. Radium and radioactive isotopes can be consumed orally or intravenously to emit alpha particles into surrounding tissues. Beta particles are small, negatively charged electrons and are used in electron beam therapy (e.g., treatment of mycosis fungoides), and can penetrate up to 1 cm of tissue. Gamma rays are uncharged photons produced by the natural decay of radioactive materials (radium, cobalt 60, etc.) and can penetrate deeply into tissues. Roentgen rays (X-rays) are similar to gamma rays, except that they are artificially emitted from tungsten when bombarded with electrons.
Radiation doses are measured in a variety of units. These units measure the energy absorbed from a radiation source per unit mass of tissue. The current unit of measure for therapeutic radiation is the Gray (Gy). The Gray is defined as the absorption of 1 J of ionizing radiation by 1 kg of tissue. The older term for this is the “rad,” and 1 rad is equal to 0.01 Gy. A typical curative treatment could be in the range of 60 to 80 Gy. Generally, adjuvant treatments tend to be within the 40 to 60 Gy range. The type of tumor, area of treatment, and goal of treatment determine the precise dosing. The total treatment is usually divided (fractionated) over the course of several sessions. This generally allows the normal, or non-diseased, tissue that surrounds the tumor, to recover better than if it were treated with one large dose.
The two main forms of radiation exposure are irradiation and contamination. Irradiation refers to radiation waves that pass directly through the human body, whereas contamination is contact with and retention of radioactive material. Contamination is usually the result of an industrial accident. The plastic surgeon is most concerned with irradiation as opposed to contamination since current regulations have made industrial accidents and exposures quite rare.
Irradiation is a local therapy applied to a specific body site containing a tumor or disease process, or to draining lymph node beds thought to contain or potentially contain microscopic or gross disease. Large tumors may be treated preoperatively with radiation therapy (induction therapy) to decrease the tumor burden prior to surgical extirpation. Adjuvant radiation therapy is performed in addition to the surgical extirpation with the goal of treating the tumor’s resection bed and regional lymph nodes in specific clinical scenarios, such as large tumors, recurrent tumors, extracapsular lymph node involvement, and positive resection margins. The potential advantage of radiation therapy over surgery is local treatment of disease with preservation of surrounding uninvolved structures. Disadvantages include the length of treatment, the need for access to appropriate facilities and equipment, and the potential additive and chronic effects of radiation therapy.
DELIVERY OF RADIATION
There is a distinction between diagnostic and therapeutic radiation. The most common application of diagnostic radiation is a simple radiograph (X-ray). The amount of radiation delivered for a standard radiograph typically ranges from 20 to 150 kV, whereas a therapeutic treatment range is typically from 200 kV to 25 MV. Radiation therapy can be delivered via external or internal routes. The delivery technique most commonly used is external beam radiotherapy, which originates from a source external to the patient, a linear accelerator (LINAC). A variety of radiation beams can be delivered in this manner, such as low-energy radiation beams from a cobalt source in a cobalt machine. Other atomic particles, such as neutrons, are also delivered via this mechanism. This technique allows daily fractionated delivery of radiation over a several week course. External beam therapy can be delivered as an independent treatment preoperatively, intraoperatively, or postoperatively.
Delivery of radiation from within the patient’s body is termed brachytherapy. Radioactive sources are inserted into the patient for temporary or permanent irradiation. This technique allows for continual treatment of the tumor with radiation over a course that usually lasts several days. Its advantages include decreased treatment time and greater ability to spare uninvolved local tissues. Brachytherapy may also be indicated in patients who have been previously irradiated and are no longer candidates for external beam therapy having already received the maximum recommended dose for a specific anatomic area. Brachytherapy is commonly used for the treatment of pelvic cancers such as cervix or prostate, and this can also be used as an adjunctive therapy for soft tissue tumors. For example, a patient may undergo external beam radiation for the treatment of a sarcoma with subsequent resection and placement of brachytherapy catheters for localized, direct radiation treatments. Plastic surgeons may be consulted because brachytherapy catheters can be covered with a soft tissue flap where primary closure is not possible. The catheters are then “loaded” with various radioisotopes. The “loaded” catheters then create a controlled, localized irradiation until the catheters are removed.
Radiation may also be delivered via robotic methods allowing for the controlled delivery of low dosages of radiation to specific anatomic locations. This technique is used for intracranial tumors, for example.
RADIATION DAMAGE
Regardless of the delivery technique, radiation therapy works by damaging the targeted cells through complicated intracellular processes whose mechanisms continue to be studied to this day. The interaction of radiation with water molecules within the cell creates free radicals that cause direct cellular damage. A range of biochemical lesions occur within DNA following exposure to radiation, and this can result in two
different modes of cell death: mitotic (clonogenic) cell death and apoptosis. The biochemical lesion most often associated with cell death is a double-stranded break of nuclear DNA.2
different modes of cell death: mitotic (clonogenic) cell death and apoptosis. The biochemical lesion most often associated with cell death is a double-stranded break of nuclear DNA.2
Irradiated tissues suffer both early and late effects. Early effects occur during the first few weeks following therapy and are usually self-limited. They result from damage to rapidly proliferating tissues, such as the mucosa and skin. Erythema and skin hyperpigmentation are the most common problems and these are treated expectantly with moisturizers, local wound care, and observation. Dry desquamation occurs after low to moderate doses of radiation, while higher doses result in moist desquamation. At the tissue level, stasis and occlusion of small vessels occur, with a resulting decrease in wound tensile strength. Fibroblast proliferation is inhibited and may result in permanent damage to fibroblasts. This creates irreversible injury to the skin which may be progressive. While the plastic surgeon is often not required to treat early radiation injuries, chronic injuries frequently require the plastic surgeon’s attention.
Late, or chronic, radiation effects can manifest anytime after therapy, from weeks to years to decades after treatment. While acute effects are uncomfortable and bothersome to the patient, they are generally self-limited and resolve with minimal treatment and local wound care. Chronic effects, however, can be progressive, disabling, cumulative, permanent, and even life threatening. Late injuries include but are not limited to tissue fibrosis, telangiectasias, delayed wound healing, lymphedema (as the result of cutaneous lymphatic obstruction), ulceration, infection, alopecia, malignant transformation, mammary hypoplasia, xerostomia, osteoradionecrosis, and endarteritis. Long-term effects of radiation therapy also include constrictive microangiopathic changes to small- and medium-sized vessels,3 which are significant when performing reconstructive procedures with either pedicled flaps or free tissue transfers.
GENERAL PRINCIPLES OF TREATING IRRADIATED WOUNDS
In most circumstances, a radiated wound will not heal as well as a nonirradiated wound. The plastic surgeon will generally be called upon to care for three different populations of irradiated patients. The first population is those who have not yet received irradiation but will be receiving radiation therapy intraoperatively or postoperatively. This is often seen in the immediate breast reconstruction patient who is undergoing mastectomy and potential postoperative radiation therapy or the sarcoma patient undergoing extirpation with intraoperative radiation therapy. Also, bronchial stumps can be reinforced when a completion pneumonectomy is anticipated, usually with intrathoracic transposition of a serratus muscle flap.4
The second patient population includes those who have already received radiation therapy and now have a recurrent or new tumor, or a radiated wound not amenable to primary closure, frequently with the exposure of vital or significant structures such as the bone, viscera, and neurovascular bundles. These patients will require tumor extirpation or wound debridement(s) followed by reconstruction.
The third group of patients includes those who require reconstruction for intraoperative radiation therapy. Intraoperative radiation therapy is occasionally used in the treatment of sarcomas, pelvic tumors, and other malignancies. In this situation, the reconstructive ladder is applicable and if reasonably healthy soft tissue is present, a primary layered closure can be attempted. Many of these wounds will heal well even though they have received intraoperative radiation therapy. However, if the bone, prosthetic material, or neurovascular bundles are exposed or if a significantly sized soft tissue defect is present, flap coverage is indicated to protect these structures and fill the defect. A subset of this patient category includes those who are receiving brachytherapy catheters intraoperatively, which require coverage.
When confronted with a wound that has late radiation changes, the first step is to rule out the presence of a recurrent or new tumor (possibly radiation induced). It is imperative that the plastic surgeon does not assume this has been ruled out by the referring physician or surgeon. Diagnosis is often assisted by standard radiographs, computed tomography (CT) scans, and magnetic resonance imaging (MRI) and is confirmed with a tissue biopsy. If tumor is present, a full workup and evaluation by the appropriate extirpative surgeon are required. After tumor extirpation is complete, reconstructive efforts of the resulting defect are then initiated.
If tumor is not present, the next step in management is complete resection and debridement of all nonviable irradiated tissues and foreign bodies (sternal wires, previous sutures, etc.).5 Primary closure or skin grafting of the irradiated wound will fail because of the poor vascularity and fibrosis of the wound bed. Likewise, muscle flaps transposed into an irradiated, poorly vascularized wound bed may not heal well. It is imperative that the plastic surgeon first establishes a clean wound with well-vascularized edges before proceeding with reconstruction. This frequently requires multiple debridements rather than a single operative endeavor, as the extent of radiation injury often exceeds what appears to be the boundary of damaged tissue. A common cause of recurrent infections, sinus tracts, and non-healing wounds is retention of nonviable materials such as foreign bodies, bone, and cartilage secondary to inadequate debridement.
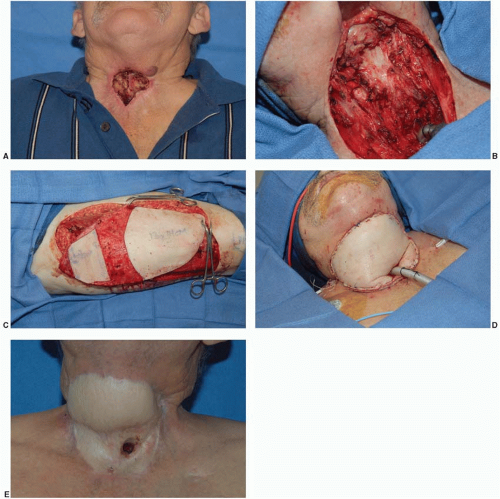
When incising severely irradiated tissue, a defect much larger than anticipated is often created. Irradiated tissue is often tight and creates a constricted skin envelope. When incised, the wound edges will retract and create a larger defect than expected (Figure 17.1). This is an important concept to understand when planning the reconstruction, as one may need more nonirradiated tissue for reconstruction than originally estimated.
Once debridement is complete, stable wound closure is obtained. Thorough preoperative planning and a systematic approach to reconstruction of irradiated defects are needed. Reconstruction usually includes transposition of a well-vascularized nonirradiated soft tissue flap. Reconstruction of these defects is often challenging and is associated with relatively high complication rates. While planning the reconstruction, the plastic surgeon chooses the flap that will best provide a healed wound and maximize preservation of function. It is generally accepted that irradiated muscles should not be transferred as this may result in partial or complete muscle necrosis.6 The transfer of a muscle whose pedicle has been irradiated may also be associated with a higher than normal complication rate.7 If a nonirradiated muscle flap or the greater omentum is not available, a free tissue transfer will be required. Since the tissue surrounding an irradiated wound is fibrotic with endothelial damage in the local vessels, the plastic surgeon must frequently ride the “reconstructive elevator” (rather than the ladder) and proceed directly with a free tissue transfer.
An important concept is that the poorly vascularized peripheral tissue surrounding the open wound requires reconstruction in addition to the wound itself. It is equally important to evaluate the tissue surrounding the defect. The flap must be approximated with well-vascularized tissue rather than irradiated, fibrotic tissue. The redundant flap may also be buried beneath the surrounding injured skin, reconstructing the missing or fibrotic subcutaneous tissue layer. This delivers additional blood supply to the skin and increases “mobility” as well. Flap coverage may also provide some pain relief for these patients. The remainder of this chapter addresses the pertinent issues of irradiated wound treatment by anatomic area.
Skin
Non-melanoma skin malignancies can be treated with approximately a 90% cure rate with irradiation (Chapter 14). Since surgical extirpation and radiation
treatment provide similar results for skin cancers, the pros and cons of each are considered before a recommendation is made. Surgical extirpation has an immediate result, whereas radiation therapy requires prolonged therapy as well as access to radiation therapy facilities. Long-term complications such as fibrosis, ulceration, ectropion, osteitis, and chondritis are possible complications of radiation therapy. It is therefore generally reserved for patients who are not surgical candidates. There is another subgroup of patients, such as those with positive cutaneous margins or perineural invasion, who may require treatment with postoperative radiation.
treatment provide similar results for skin cancers, the pros and cons of each are considered before a recommendation is made. Surgical extirpation has an immediate result, whereas radiation therapy requires prolonged therapy as well as access to radiation therapy facilities. Long-term complications such as fibrosis, ulceration, ectropion, osteitis, and chondritis are possible complications of radiation therapy. It is therefore generally reserved for patients who are not surgical candidates. There is another subgroup of patients, such as those with positive cutaneous margins or perineural invasion, who may require treatment with postoperative radiation.
Low-dose radiation therapy is also used postoperatively in the treatment of benign disease, such as keloids and hypertrophic scars. This technique takes advantage of fibroblast inhibition caused by ionizing radiation. The radiation is generally administered on the same day the keloid is excised and for several days thereafter.
Extremities
Soft tissue sarcomas of the extremities can be aggressive tumors involving multiple structures and tissue planes. Surgical extirpation is often combined with intraoperative or postoperative radiation therapy, either external beam or brachytherapy. Therefore, treatment of these patients requires a multidisciplinary approach often involving surgical oncologists, vascular surgeons, orthopedic surgeons, radiation oncologists, plastic surgeons, and others (Chapter 94). The goal is to obtain locoregional tumor control while simultaneously attempting limb salvage and maximal preservation of limb function. Patients may have received irradiation before extirpation, which is important in the planning of the radiation therapy (i.e., the patient may require brachytherapy as opposed to external beam therapy or a modification of the external beam dose). The sequence is especially important to the plastic surgeon and the planning of wound closure and reconstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree