Pustular Eruptions of Palms and Soles: Introduction
|
Pustular eruptions of the palms and soles include palmoplantar pustulosis (PPP), acrodermatitis continua (Hallopeau disease), and infantile acropustulosis. The entities present with chronic and persistent eruptions of sterile, purulent vesicles.
Palmoplantar Pustulosis
PPP is a chronic pustular dermatosis localized on the palms and soles only. High resistance to treatment and a high recurrence rate are characteristic. Histologically, it is characterized by intraepidermal vesicles filled with neutrophils.
PPP has a worldwide distribution. It is a rare condition, but the exact incidence is not known. Females show a higher prevalence than males, with a ratio of approximately 3:1. Onset of the disease occurs mostly between the ages of 20 and 60 years; rarely, the condition occurs after the sixth decade of life, and in 10% of the patients the onset is before the age of 20 years.
HLA typing of patients with PPP reveals no increased frequency of any of the known psoriasis-linked alloantigens.1 In a direct comparison among chronic-plaque psoriasis, guttate psoriasis, and PPP, the three major candidate genes within the PSORS1 region [(1) HLA-Cw*6, (2) HCR*WWCC, and (3) CDSN*5] showed a high association to guttate and chronic plaque psoriasis, not, however, to PPP.2 Investigation of the apolipoprotein E alleles e2, e3, and e4 in chronic plaque and guttate psoriasis as well as in PPP in acitretin responders and nonresponders showed that the frequency of the e4 allele was significantly higher in the psoriasis groups but not in PPP patients as compared to healthy controls.3 In chronic plaque psoriasis and psoriatic arthritis an association with TNF-α-238 and -308 promoter polymorphisms have been found; however, the association has not been found in PPP.4 Studies from Japan provide evidence for phenotypic and genetic heterogeneity of PPP according to its association/provocation with tonsillitis. In patients in whom PPP was not associated with tonsillitis, the phenotype frequency of the TNF-β2 allele of the TNF-β gene and of the allele B of the TNF-α gene was significantly higher as compared to controls.5
Genetic association studies in a Caucasian cohort revealed that genes encoding for cytokines of the IL-10 family, namely, IL-19, IL-20, and IL-24 show haplotypes conferring increased risk for PPP.6
These findings further substantiate the notion that PPP and psoriasis represent different entities.
The cause of PPP is not known. An imbalance of the protease/antiprotease system in the skin consisting of decreased antileukoprotease (elafin/SKALP) activity in pustular psoriasis has been discussed as a possible mechanism of pustule formation.7 Exacerbation of PPP has been observed after patch testing with metals and was accompanied by elevated leukotriene B4 levels in plasma and pustules.8
In a long-term survey from Japan, the incidence of PPP was found to be positively correlated to heavy smoking (more than 20 cigarettes per day), tonsillitis, and seasonal factors such as high humidity and high temperature.9,10
The most striking association in PPP is smoking. In two Swedish surveys, 95% of PPP patients were smokers at onset of disease and cessation of smoking was the most important measure to treat the disease.11,12 There is evidence from immunohistochemical studies that nicotinic acetylcholine receptors are modulated in lesional skin from smoking PPP patients when compared to disease-free smokers and healthy controls suggesting an abnormal response to nicotine in PPP.13 The possible involvement of neutrophils infiltrating sweat glands and ducts expressing choline acetyltransferase and the α-3 and α-7 nicotinic acetylcholine receptors as a target for nicotine/smoking was discussed as an important mechanism for manifestation and/or maintenance of PPP.14
Investigating the tissue of tonsils of PPP-patients a unique formation of lymphoid follicles surrounded by reticular crypt epithelial cells was found which was absent in tonsils of controls.15 Culturing crypt epithelial cells it was shown that p53-related expression factors contributed to an upregulation of IL-6 gene expression. The possible importance of IL-6 for PPP has been emphasized previously and it has been shown that tonsillectomy leads to improvement of lesions.16 In another study, the expression of inducible costimulator (ICOS), a costimulatory receptor on activated T cells was higher in the tissue of tonsils of PPP-patients as compared to controls.17 Tonsillectomy or treatment of dental foci resulted in a marked and sustained improvement of lesions suggesting a major role of focal infections as a trigger for PPP. The role of T cells in the tonsils for PPP is further substantiated by the demonstration of an increased expression of cutaneous lymphocyte-associated antigen (CLA) on CD3+ T cells in tonsils and in diseased skin together with an enhanced expression of the CLA-ligand E-selectin.18
Grafting involved PPP-skin onto SCID/CB-17 mice injected with lymphocytes from tonsils of PPP patients together with heat shock protein 60 induced high antiheat shock protein 65-IgG levels together with an increase of IL-6 and interferon α.19 Recruitment of lymphocytes seems to be mediated by the chemokine CCL20/MIP3 α the receptor of which, CCR6, is significantly expressed on tonsilar T cells of PPP patients as compared to controls. Indeed, Tonsillectomy resulted in a decreased CCR6 expression on peripheral PPP T cells.20
The observation of either PPP or new onset psoriasis in patients treated with anti-TNF-α agents is not yet understood21 but a shift from a TNF-α-driven immune response toward an interferon-dominated inflammatory response is discussed.22 In an animal model, the neutralization of TNF-α-induced skin inflammation resulted in an increased expression of IL-1b, IL-6, IL-17, IL-21, and IL-22 and a suppression of FoxP3-positive regulatory T cells.23 In the light of the importance of T cells and IL-6 for the development of PPP this shift may at least in part explain this appears relevant.
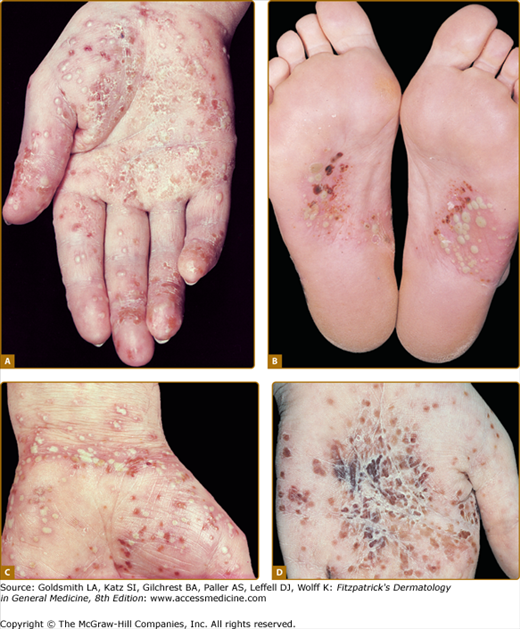
The primary lesions are pustules of nearly equal size measuring two to four mm in diameter. Crops of pustules usually arise within a few hours on otherwise normal-appearing palmar and plantar skin (Fig. 21-1). Involvement is usually symmetric but unilateral location on palms and/or soles can be seen. Single lesions then become surrounded by an erythematous ring. Sometimes, the pustules extend to the dorsa of the fingers, the feet, or over the volar wrists (see Fig. 21-1C). Episodes of new pustular eruptions occur at varying intervals and remain strictly confined to the sites of predilection.
Figure 21-1
Palmoplantar pustulosis. A and B. Groups of pustules measuring 2 to 4 mm in diameter occur on erythematous skin on palms and soles. Both feet and both hands are normally affected symmetrically but can also be found on one side only. C and D. Lesions may occasionally spread beyond the predilection sites, and pustules may appear on the wrists. Within several days after pustule formation, lesions dry, flatten, and acquire a brownish color. This may be followed by eczematous changes with scaling and fissuring.
As pustules become older, their yellow color changes to dark brown, so that in untreated PPP, the lesions show various shades of color (see Fig. 21-1). Dried pustules are shed within approximately 8 to 10 days.
Symptoms include itching or a burning sensation, which may precede new crops of lesions. However, in severe eruptions, pain and the inability to stand, walk, or do manual work may greatly reduce the quality of life.
(Box 21-1.) An association of PPP and osteoarthritis of the anterior chest wall was first described in Japan.24 As reported by Swedish authors, involvement of the manubriosternal joint is present in 6% and of the sternoclavicular joints in 10% of patients.25 Scintigraphic investigations showed sternocostoclavicular joint involvement to be present in 16 of 73 (22%) patients.26 For this condition, the term SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) has been established.27 Clinical manifestations of SAPHO syndrome are similar when they occur either with severe acne (mostly acne conglobata) or PPP (see Chapter 80). The primary lesion consists of a sterile abscess containing neutrophils. The site of predilection is the anterior chest wall. Involvement of the sacroiliacal joints may occur.28
PPP is also seen in patients with chronic recurrent multifocal osteomyelitis as well as with noninfectious inflammatory bone lesions.
An association of PPP with gluten-sensitivity has been suggested as far back as 1991.29 In a more recent study of 123 patients with PPP IgA-antibodies against gliadin were found in 18% of patients and against tissue transglutaminase in 10%, respectively.30 In these patients CD3+ and CD8+ T cells were increased in numbers in duodenal biospsies. In 6% of patients diagnosis of celiac disease was made. Patients who tested positive for any of the antibodies showed total or nearly total clearance of skin lesions when they adhered to a gluten-free diet.
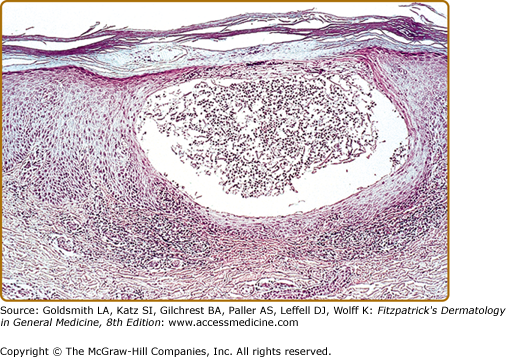
Histologically, there is an intraepidermal cavity filled with polymorphonuclear leukocytes associated with spongiform changes within the surrounding epidermis (Fig. 21-2). Eosinophils and mast cells are present in increased numbers in PPP biopsies from lesional skin. Another hallmark is the inability to visualize the epidermal part of the eccrine duct in PPP specimens indicating an involvement of the acrosyringium.31
The lesions of PPP are sterile; a moderately increased white blood cell count may occasionally be observed, but all other laboratory tests are usually normal. In patients with an infectious trigger, infection-associated laboratory parameters, such as C-reactive protein, may be increased. Increased levels of antigliadin and/or tissue transglutaminase antibodies may be found.
(Box 21-2)
Most Likely
|
Consider
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree