Psoriatic Arthritis: Introduction
|
Definition and Classification
Psoriatic arthritis (PsA) is an inflammatory musculoskeletal disease that affects people with psoriasis or their near relatives. It affects musculoskeletal structures such as the peripheral and axial joints, entheses, and tendon sheaths. The eye and mucous membranes are also often involved. Thus, the disease has varied manifestations that make diagnosis and assessment sometimes difficult.
The original case definition for PsA was provided by Moll and Wright in 1973.1 They defined PsA as an inflammatory arthritis associated with cutaneous psoriasis, seronegative for rheumatoid factor. Rheumatoid factor is a marker for rheumatoid arthritis (RA); thus, the definition was meant to help distinguish PsA from RA, which at that time was a more recognized form of inflammatory arthritis. The CASPAR study group recently developed a new set of criteria for classification of PsA using data collected prospectively in patients with long-standing disease (Box 19-1).2 The CASPAR criteria had specificity of 98.7% and sensitivity of 91.4% in the original study, with excellent sensitivity in both early and late disease. The criteria allow classifying patients even when they do not have current, past, or family history of psoriasis and are now used in epidemiologic and genetic studies in PsA.
To meet the CASPAR (ClASsification criteria for Psoriatic ARthritis) criteria*, a patient must have inflammatory articular disease (joint, spine, or entheseal) with three points from the following categories:
|
Historical Aspects
![]() The co-occurrence of cutaneous psoriasis with arthritis was first reported by the pioneering French dermatologist Baron Jean-Louis Marc Alibert in the year 1818.3 However, it was only in 1964 that the American Rheumatism Association recognized this condition as a distinct clinical entity. Even after this recognition research and assessment of this clinical condition did not attract much attention save for a few active research groups, especially in Leeds, UK and Toronto, Canada. John Moll and Verna Wright from Leeds provided a disease definition and described five disease patterns.1 They also carried out the seminal genetic epidemiologic study proving a strong genetic basis for PsA.4 Since PsA manifests peripheral arthritis as well as axial arthritis, assessment tools and response criteria for PsA were borrowed from RA and ankylosing spondylitis (AS).5 Moreover, since most if not all patients with PsA have cutaneous (mostly chronic plaque) psoriasis, assessment and response criteria for psoriasis have been included in the assessment of PsA.5 With the advent of therapy with anti-TNF agents, it was realized that the disease definition and assessment tools were inadequate. Subsequently, researchers with an interest in PsA successfully formed the ClASsification criteria for Psoriatic ARthritis (CASPAR) Study Group to work toward a better case definition. This group was then expanded to include dermatologists, methodologists, and geneticists to form a larger international research group–the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)—leading to new developments in assessment and treatment of PsA.6
The co-occurrence of cutaneous psoriasis with arthritis was first reported by the pioneering French dermatologist Baron Jean-Louis Marc Alibert in the year 1818.3 However, it was only in 1964 that the American Rheumatism Association recognized this condition as a distinct clinical entity. Even after this recognition research and assessment of this clinical condition did not attract much attention save for a few active research groups, especially in Leeds, UK and Toronto, Canada. John Moll and Verna Wright from Leeds provided a disease definition and described five disease patterns.1 They also carried out the seminal genetic epidemiologic study proving a strong genetic basis for PsA.4 Since PsA manifests peripheral arthritis as well as axial arthritis, assessment tools and response criteria for PsA were borrowed from RA and ankylosing spondylitis (AS).5 Moreover, since most if not all patients with PsA have cutaneous (mostly chronic plaque) psoriasis, assessment and response criteria for psoriasis have been included in the assessment of PsA.5 With the advent of therapy with anti-TNF agents, it was realized that the disease definition and assessment tools were inadequate. Subsequently, researchers with an interest in PsA successfully formed the ClASsification criteria for Psoriatic ARthritis (CASPAR) Study Group to work toward a better case definition. This group was then expanded to include dermatologists, methodologists, and geneticists to form a larger international research group–the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)—leading to new developments in assessment and treatment of PsA.6
Epidemiology
![]() The prevalence estimates of PsA vary depending on the study methodology and the geographic location.7 Recent studies from Europe estimate a population prevalence of 0.06%–0.42%, while those from the United States, estimate a prevalence of 0.158%–0.25%. Estimates of prevalence of PsA in patients with psoriasis vary widely, ranging from 6% to 42%. Population-based studies report prevalence estimates between 6% and 13.8%, whereas, clinic-based studies have reported higher PsA prevalence ranging from 18.2% to 42%. Thus, patients evaluated in dermatology practices have a higher prevalence of PsA, compared to those in the community.
The prevalence estimates of PsA vary depending on the study methodology and the geographic location.7 Recent studies from Europe estimate a population prevalence of 0.06%–0.42%, while those from the United States, estimate a prevalence of 0.158%–0.25%. Estimates of prevalence of PsA in patients with psoriasis vary widely, ranging from 6% to 42%. Population-based studies report prevalence estimates between 6% and 13.8%, whereas, clinic-based studies have reported higher PsA prevalence ranging from 18.2% to 42%. Thus, patients evaluated in dermatology practices have a higher prevalence of PsA, compared to those in the community.
![]() There are fewer studies on the incidence of PsA. The incidence in Europe and North America range between 3 and 23.1/100,000, whereas that in Japan was only 0.1/100,000. A population-based study from Minnesota, USA from the Rochester Epidemiology Project showed that the overall annual incidence of PsA was 7.2/100,000. Interestingly, the incidence increased significantly from 3.6 in 1970–1979 to 9.8 in 1990–2000. Incidence in patients with psoriasis is less well known. The Rochester epidemiological project reports a cumulative incidence of 1.7%, 3.1%, and 5.1% at 5, 10, and 20 years following psoriasis incidence, respectively. The likelihood of PsA in psoriasis subjects did not change over time. An international clinic-based study from Europe similarly showed the incidence of PsA among psoriasis patients remained constant (74 per 1,000 person-years).
There are fewer studies on the incidence of PsA. The incidence in Europe and North America range between 3 and 23.1/100,000, whereas that in Japan was only 0.1/100,000. A population-based study from Minnesota, USA from the Rochester Epidemiology Project showed that the overall annual incidence of PsA was 7.2/100,000. Interestingly, the incidence increased significantly from 3.6 in 1970–1979 to 9.8 in 1990–2000. Incidence in patients with psoriasis is less well known. The Rochester epidemiological project reports a cumulative incidence of 1.7%, 3.1%, and 5.1% at 5, 10, and 20 years following psoriasis incidence, respectively. The likelihood of PsA in psoriasis subjects did not change over time. An international clinic-based study from Europe similarly showed the incidence of PsA among psoriasis patients remained constant (74 per 1,000 person-years).
Etiology and Pathogenesis
PsA has a strong genetic component. The estimated recurrence risk ratio (λ) in first-degree relatives (FDRs) of probands with PsA ranges from 30.4 to 55, indicating a high genetic contribution to disease susceptibility.8 Strong heritability was also demonstrated in a recent study from Iceland where patients known to have PsA in Reykjavik were linked to the Icelandic genealogy database.9 FDRs to fourth-degree relatives of patients with PsA had relative risks of 39, 12, 3.6, and 2.3, respectively, reflecting a strong genetic component. The decrease of λ-1 more rapidly than by a factor of 2 with the degree of relationship indicates that multiple genes contribute to susceptibility with some interaction of effects. Genes associated with PsA include HLA alleles, MHC Class I related chain (MIC) genes, TNFA, IL23R, IL1, and killer-cell immunoglobulin-like receptor (KIR) genes. HLA-B13, -B16 and its splits -B38 and -B39, -B17, and -Cw6 are associated with psoriasis, with or without arthritis, -B27 and -B7 are specifically associated with PsA.8 However, since most patients with PsA have cutaneous psoriasis it is difficult to determine whether genetic associations found in patients with PsA when compared to healthy controls is associated with psoriasis or with PsA. A recent genome-wide association study was able to investigate association with PsA and differences between PsA and psoriasis alone.10 HLA-C, IL12B, and TNIP1 were associated with PsA when compared to normal controls. There was a statistically significant difference between PsA and psoriasis alone at three loci [(1) HLA-C, (2) IL12B, and (3) IL23R]. HLA-C and IL23R were more strongly associated with psoriasis alone, and IL12B with PsA. A smaller GWAS also identified a novel PsA (and potentially psoriasis) locus on chromosome 4q27 that harbors the interleukin 2 (IL2) and interleukin 21 (IL21) genes.11
Genetic polymorphisms can also influence PsA phenotype. HLA-B39 alone, HLA-B27 in the presence of HLA-DR7, and HLA-DQ3 only in the absence of HLA-DR7, confers increased risk for disease progression.12 TNF polymorphisms are associated with erosive disease and joint damage progression in early PsA.13 The interleukin-4 receptor gene (IL4R) I50V SNP is associated with erosive PsA, although the association was not consistently shown.14,15
![]() In complex genetic disease like PsA, both genetic and environmental factors influence disease susceptibility. Associations with Rubella vaccination, injury sufficient to require a medical consultation, recurrent oral ulcers, moving house, bone fractures requiring hospital admission, HIV infection, and corticosteroid use have been reported.16 Smoking, psoriasis, and PsA have an interesting relationship. The time to development of PsA decreases with smoking prior to psoriasis onset, but the time to development of PsA increases with smoking after psoriasis onset.17 IL13 gene polymorphisms are associated with protection from PsA. However, smoking abrogates this protective effect.
In complex genetic disease like PsA, both genetic and environmental factors influence disease susceptibility. Associations with Rubella vaccination, injury sufficient to require a medical consultation, recurrent oral ulcers, moving house, bone fractures requiring hospital admission, HIV infection, and corticosteroid use have been reported.16 Smoking, psoriasis, and PsA have an interesting relationship. The time to development of PsA decreases with smoking prior to psoriasis onset, but the time to development of PsA increases with smoking after psoriasis onset.17 IL13 gene polymorphisms are associated with protection from PsA. However, smoking abrogates this protective effect.
The immune system, especially the lymphocytes, play an important role in the pathogenesis of PsA. The traditional model of the pathogenesis is that autoimmunity directed against a common skin and joint autoantigen(s) leads to chronic autoreactive T-cell driven inflammation. Genetic susceptibility predisposes the T-cell receptor repertoire to recognition of target self-peptides expressed in target tissues. Prior response to exogenous ligands encoded by pathogens as well as prior episodes of inflammation result in expansion of memory effector CD8+ T cells that recognize stress-related self-antigens and initiates and maintains pathways of inflammation mediated by the expression of transcription factors such as nuclear factor-κB and activator protein-1, resulting in skin and synovial inflammation.18 The primary defects in this model involve expression of an autoantigen, binding of autoantigen peptides by MHC Class 1 molecules leading to initial clonal activation and expansion of an adaptive immune response. However, recent imaging, histological, and genetic studies have made us reconsider this view, especially with respect to joint and nail diseases. Clinically unrecognized enthesitis is commonly seen in PsA and in psoriasis without arthritis.19 Enthesitis is associated with adjacent osteitis or bone and synovial inflammation. Nail disease is a marker for PsA in patients with psoriasis, since it occurs in almost 90% of patients with PsA, but less than 50% of those with psoriasis alone. The nail is closely related to the distal interphalangeal (DIP) joints and the related extensor tendon insertion sites, and the association between DIP joint disease, adjacent nail lesions, and entheseal inflammation was recently demonstrated.20 It is proposed that the synovial membrane and entheses form a “synovioentheseal complex,” and that enthesitis is the unifying pathologic lesion that may explain the varied clinical manifestations of PsA.21 In this model, tissue specific factors, including microtrauma, lead to regional innate immune activation and persistent inflammation. The genetic association of PsA with Class 1 HLA alleles and KIR genes as well as the association between juvenile PsA and Mediterranean Fever (MEFV) and NLR family, pyrin domain containing 3 (NLRP3) genes indicate that PsA is closer to the “autoinflammatory” end rather than the “autoimmune” end of the spectrum of immune-mediated diseases.22 Disease localization in autoinflammatory diseases is determined predominantly by the innate immune response to local tissue specific factors.
There is also evidence that the monocyte–macrophage system plays a major role in the initiation and perpetuation of joint inflammation. Monocytes differentiate into macrophages, osteoclasts, Langerhans cells, or dendritic cells in response to microenvironmental signals.23 In entheseal tissues, monocytes are the principal cells that infiltrate fibrocartilage. Monocytes are also present in the synovial lining of psoriatic joints, and they infiltrate the subsynovial lining. An increased frequency of circulating osteoclast precursors was identified in the circulation and synovial tissues of PsA patients. These precursors, derived from circulating CD14+ monocytes, differentiate into osteoclasts after exposure to monocyte colony stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) expressed by synovial lining cells in inflamed psoriatic synovium, and is responsible for bone erosions. The frequency of circulating osteoclast precursors in PsA patients decline rapidly following treatment with anti-TNF agents and may explain its antierosive effects.
![]() Synovial histopathology of PsA is characterized by lining layer hyperplasia, neovascularization, infiltration by T and B lymphocytes, plasma cells, and macrophages. The T-cell infiltrate consists of a small number of very large CD8 T-cell clonal expansion and polyclonal CD4 T-cells. The CD8 T-cell clones are derived from clonal precursor pools that are highly expanded in the blood. Their phenotype is of memory CD8 T cells that have lost the costimulatory CD28 molecule, but gained receptors that are typically expressed on and regulate the activation of NK cells. The CD4 T-cell are nonantigen specific and arise from chemokine-mediated recruitment. Cytokines that have been implicated in the pathogenesis include macrophage-derived TNF-α, interleukin (IL)-6, IL-1α, and lymphocyte-derived IL-2 and interferon-γ. Infiltrating T cells elaborate chemokines, including RANTES (regulated on activation, normal T cell expressed and secreted). The chemokine IL-8 is expressed at a high level by the synovial lining cells and to a lesser extent in cells located in the perivascular areas and likely mediates attraction of neutrophils to the joint fluid, a characteristic feature of PsA. Lining cells also elaborate the chemokine monocyte chemotactic protein 1 (MCP1/CCL2), a chemotactic factor that attracts and activates monocytes but not neutrophils. Macrophages in the lining produce chemokines such as GRO-α (growth-regulated protein α precursor, CXCL1), which also has chemotactic activity for neutrophils. Vascular abnormalities are prominent in the psoriatic synovium. These changes are likely to reflect specific cytokine profiles augmented in the psoriatic patient.
Synovial histopathology of PsA is characterized by lining layer hyperplasia, neovascularization, infiltration by T and B lymphocytes, plasma cells, and macrophages. The T-cell infiltrate consists of a small number of very large CD8 T-cell clonal expansion and polyclonal CD4 T-cells. The CD8 T-cell clones are derived from clonal precursor pools that are highly expanded in the blood. Their phenotype is of memory CD8 T cells that have lost the costimulatory CD28 molecule, but gained receptors that are typically expressed on and regulate the activation of NK cells. The CD4 T-cell are nonantigen specific and arise from chemokine-mediated recruitment. Cytokines that have been implicated in the pathogenesis include macrophage-derived TNF-α, interleukin (IL)-6, IL-1α, and lymphocyte-derived IL-2 and interferon-γ. Infiltrating T cells elaborate chemokines, including RANTES (regulated on activation, normal T cell expressed and secreted). The chemokine IL-8 is expressed at a high level by the synovial lining cells and to a lesser extent in cells located in the perivascular areas and likely mediates attraction of neutrophils to the joint fluid, a characteristic feature of PsA. Lining cells also elaborate the chemokine monocyte chemotactic protein 1 (MCP1/CCL2), a chemotactic factor that attracts and activates monocytes but not neutrophils. Macrophages in the lining produce chemokines such as GRO-α (growth-regulated protein α precursor, CXCL1), which also has chemotactic activity for neutrophils. Vascular abnormalities are prominent in the psoriatic synovium. These changes are likely to reflect specific cytokine profiles augmented in the psoriatic patient.
![]() Studies comparing the synovial histopathology of PsA, other spondyloarthritis (SpA) and RA have concluded that PsA histopathology resembles that of SpA more than it does RA.24,25 The lining layer thickness and number of T-cells (CD4 and CD8), CD83+ dendritic cells, CD38+ plasma cells are greater in RA than in SpA. Vascularity, infiltration by polymorphonuclear cells and CD163+ macrophages were higher in SpA. ICAM-1 staining in the synovial lining layer was also higher in SpA than in RA. Intracellular citrullinated proteins and major histocompatibility complex-human cartilage glycoprotein 39 (MHC-HC gp39) peptide complexes were observed only in RA. There were no differences between PsA and non-PsA SpA. Moreover, no significant differences were present between oligoarticular and polyarticular PsA. The expression of TNF-α, IL1β, IL6, and IL18 was as high in the synovium in PsA as in RA. These studies firmly establish that PsA is a SpA, and not just a co-occurrence of RA with psoriasis.
Studies comparing the synovial histopathology of PsA, other spondyloarthritis (SpA) and RA have concluded that PsA histopathology resembles that of SpA more than it does RA.24,25 The lining layer thickness and number of T-cells (CD4 and CD8), CD83+ dendritic cells, CD38+ plasma cells are greater in RA than in SpA. Vascularity, infiltration by polymorphonuclear cells and CD163+ macrophages were higher in SpA. ICAM-1 staining in the synovial lining layer was also higher in SpA than in RA. Intracellular citrullinated proteins and major histocompatibility complex-human cartilage glycoprotein 39 (MHC-HC gp39) peptide complexes were observed only in RA. There were no differences between PsA and non-PsA SpA. Moreover, no significant differences were present between oligoarticular and polyarticular PsA. The expression of TNF-α, IL1β, IL6, and IL18 was as high in the synovium in PsA as in RA. These studies firmly establish that PsA is a SpA, and not just a co-occurrence of RA with psoriasis.
Clinical Findings
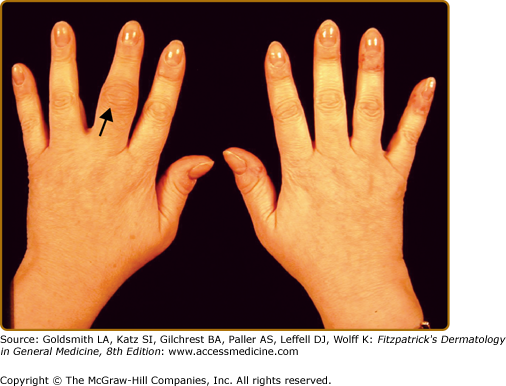
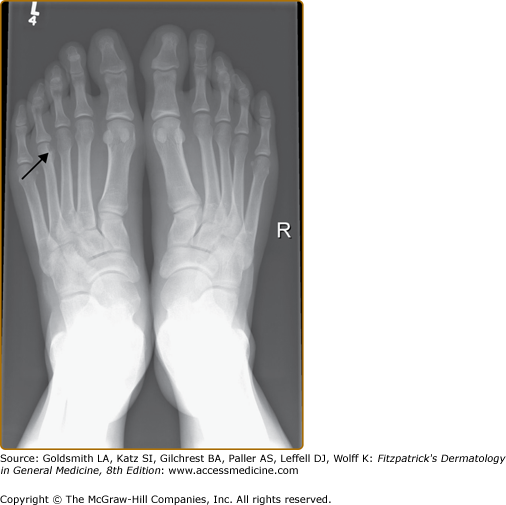
The CASPAR classification criteria define PsA as an inflammatory musculoskeletal disease that can involve the joints, spine or entheses. Clinical features of PsA include peripheral arthritis, axial arthritis (spondylitis), enthesitis, dactylitis, and tenosynovitis. The typical features of inflammatory arthritis are joint pain, swelling, stiffness, redness, and reduction in mobility. The arthritis of PsA is often of gradual onset and involves one or more joints. Typically early PsA is oligoarticular (affecting less than five joints) (Fig. 19-1). Early PsA often involves the joints of the lower limbs, but any joint of the body may be affected. Oligoarticular PsA subsequently evolves into polyarthritis. Joints that are typically affected in patients with PsA include the DIP joints (Fig. 19-2). Commonly the nails near these affected jointsdemonstrate the nail changes that are typical for psoriasis. The distribution of the joint involvement in PsA is often asymmetric. However, as the number of joints affected increases, there is a tendency toward symmetry.26 Patients with PsA have less pain than those with RA.27 Therefore, they may be oblivious to the degree of inflammation in their joint. In addition to peripheral arthritis, PsA affects the axial joints in 30%–50% of patients with PsA. Axial inflammatory arthritis presents with inflammatory back and/or neck pain, typically associated with stiffness and worse after periods of prolonged inactivity such as nighttime sleep. Inflammatory axial pain and stiffness usually improve with activity. However, evidence of radiographic involvement of the axial joints may be present in a substantial proportion of patients presenting with peripheral PsA in the absence of axial symptoms.28 Axial arthritis leads to restriction in the mobility of the spine. The process can lead to a completely fused and immobile spine, sometimes called “bamboo spine.”
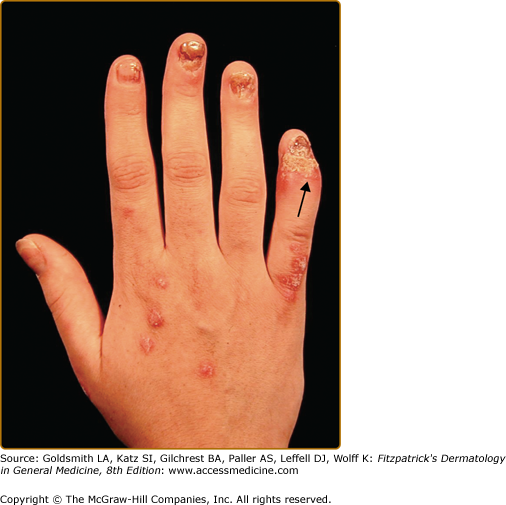
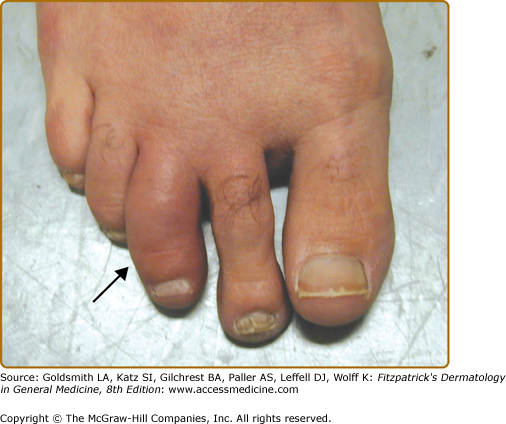
Dactylitis, defined as inflammatory swelling of an entire finger or toe, is a characteristic manifestation of PsA (Fig. 19-3). This is due to inflammation of the joints, tendons, bones, and soft tissues in the digit(s). Persistent dactylitis leads to destruction of the joints in that digit. Dactylitis is a marker of severity of psoriatic arthritis. Enthesitis, defined as inflammation at the entheses (site where ligaments or tendons attach to bone), is another important manifestation of PsA. The most common sites to be affected by enthesitis are the plantar fascia on the sole of the feet (plantar fasciitis) and Achilles tendon insertion at the back of the heel (Achilles enthesitis). Enthesitis can also affect other sites including tendon insertion sites on the patella, shoulder, elbows, pelvis, spinous processes, and chest wall. Tenosynovitis, or inflammation of the tendon sheath, may affect tendons in the hands, wrists, and around the ankles. (Box 19-2).
Five patterns of PsA were originally described by Moll and Wright.* These include the following:
|
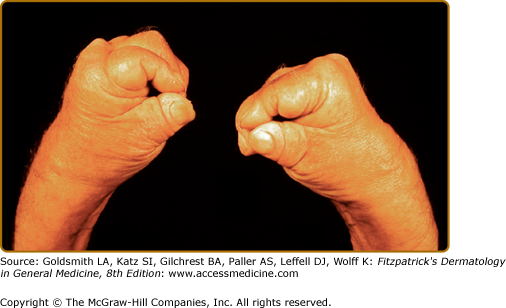
The category of asymmetric oligoarthritis includes those patients with four or less joints affected by arthritis. The joints involved are usually of the lower limbs and there is lack of symmetry. In the category of symmetric polyarthritis, five or more joints are involved in a symmetric fashion. Therefore, it is sometimes difficult to distinguish it from RA. The spondyloarthritis category includes patients with predominant involvement of the spine (axial arthritis), similar to the involvement in patients with AS. As the name suggests, the category of distal interphalangeal joint arthritis includes those patients with predominant involvement of the DIP joints. Arthritis mutilans describes a category with severe arthritis leading to shortening and destruction of fingers and toes (see eFig. 19-3.1). Although, these categories were described initially, it was soon realized that as the longer the duration of the disease the higher the number of joints involved and the involvement becomes more symmetric. Distal joint involvement also is common and is seen in all categories. Arthritis mutilans is also a manifestation of severity of the arthritis process and not an exclusive category. Therefore, experts nowadays tend to classify the disease as peripheral arthritis alone, peripheral arthritis with axial arthritis, and axial arthritis alone.29
Most patients with PsA have psoriasis vulgaris. In about 70% of people with PsA, psoriasis develops first and the arthritis manifests itself after a variable duration, on average within 10 years. However, in about 15%, both arthritis and psoriasis develops simultaneously, and in the remaining, the arthritis develops first, and cutaneous psoriasis manifests itself a few years later. Patients seen in dermatology clinics and those hospitalized with psoriasis have high prevalence of PsA compared to patients with psoriasis seen in the community. Patients reporting greater extent of psoriasis also reported a higher prevalence of PsA when surveyed in a telephone interview.30 Thus, patients with more severe psoriasis may have a higher prevalence of PsA. However, most patients attending rheumatology clinics for their arthritis have only mild to moderate psoriasis.
Psoriatic nail dystrophy usually manifests as pitting and onycholysis (see Chapter 18). Although 40% of patients with psoriasis without PsA have nail lesions, nail involvement is much more frequent in patients with PsA affecting close to 90% of patients.31 Thus, nail lesions are the only clinical feature that distinguishes patients with psoriatic arthritis from those with uncomplicated psoriasis. The nail bed is closely linked to the DIP joints; nail involvement is associated with arthritis of these joints.
Apart from the involvement of the skin, nails, and joints, people with PsA also have involvement of other important organs. Eye involvement is not infrequent. Conjunctivitis is occasionally seen and uveitis may occur in 2.3% of patients.32 Mucous membrane inflammation presents with painful mouth ulcers and urethritis. Inflammatory bowel disease in PsA may resemble Crohn disease and/or ulcerative colitis and can cause abdominal pain, loose stools, and bleeding.
Laboratory tests are usually done at diagnosis and periodically thereafter. However, there is to date no diagnostic test for PsA. The acute phase reactants [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)] are often normal. ESR and/or CRP are raised in less that 50% of people with PsA. Rheumatoid factor is usually negative and helps exclude RA. HLA-B*27 is positive in 20% of patients with PsA. Radiological investigations (X-ray, ultrasonography, and magnetic resonance imaging) can provide important clues to diagnosis and to the extent of inflammation and damage. However, only the presence of periostitis and new bone formation near joint margins may be considered reasonably specific to PsA.2 Although, these tests are not by themselves diagnostic, they help in ruling out other conditions that may mimic PsA. Synovial fluid if obtained demonstrates inflammation and helps exclude crystal arthritis and infections. Investigations such as blood count, and liver and kidney functions are often obtained to monitor side effects of drug therapy.
Imaging is an important modality in the assessment of patients with PsA. Imaging complements clinical assessment and helps in confirming the diagnosis as well as in determining disease severity. The various modalities used in assessment of PsA include X-rays, ultrasound, computerized-tomography scan (CT scan), magnetic resonance imaging (MRI), and bone scan.
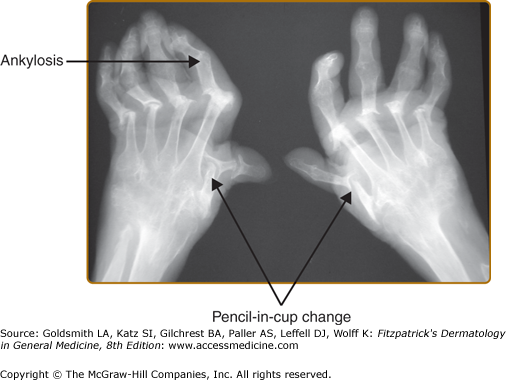
X-rays are the mainstay in the radiologic assessment of PsA. X-rays are relatively cheap, easily available, and can be read by most physicians. Once PsA is suspected clinically, radiographs of the hands, feet, pelvis, spine, and other affected joints are done to look for changes suggestive of PsA. X-rays are also used to assess disease severity, as well as to follow disease progression. Radiographic changes generally reflect damage to the joints rather than acute inflammation. In early disease, X-rays of the hands and feet show soft tissue swelling around the joints involved. If dactylitis is present, soft tissue swelling will involve the whole finger or toe. In more severe disease, erosions develop near the joint margin and are markers of disease severity (Fig. 19-4). Erosions may be paramarginal, in contrast to RA were the erosions are usually marginal. In PsA erosions are often accompanied by new bone formation. The combination of erosions and new bone formation at joint margins is characteristic of PsA (Fig. 19-5). Following the development of erosions there may be joint space narrowing, and finally total joint destruction may occur, either total joint lysis and the so called “pencil-in-cup” change or complete bony bridging through the joint termed ankylosis (Fig. 19-6).
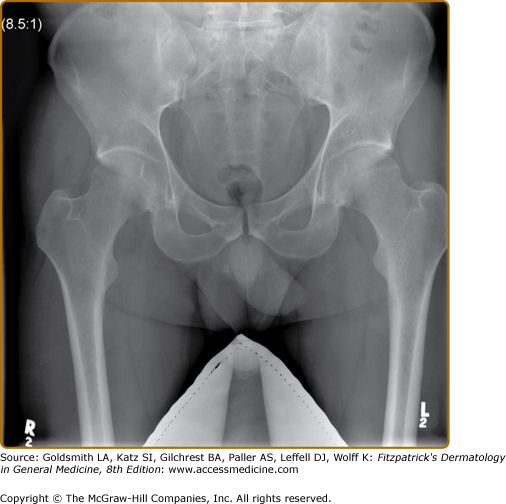
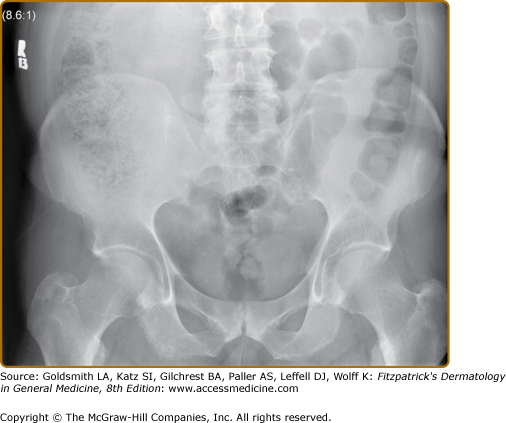
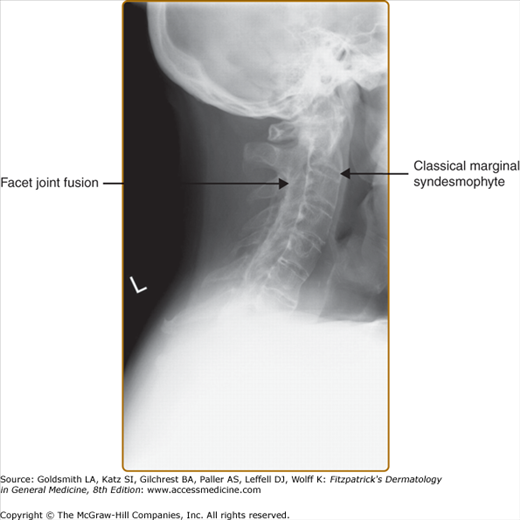
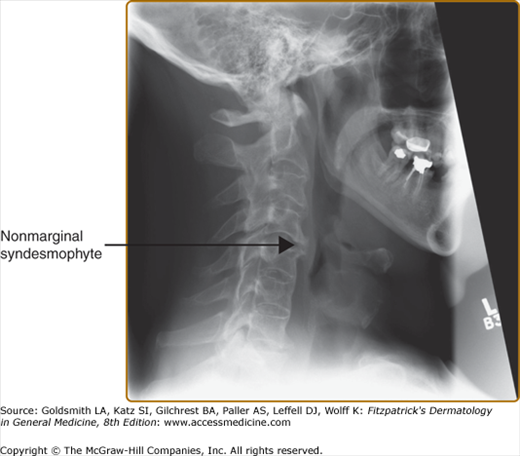
X-rays of the pelvis often show changes reflecting the presence of sacroiliitis. Earliest notable changes include widening of the joint space, which is often difficult to appreciate (eFig. 19-6.1). Subsequently, erosions develop, followed by sclerosis and subsequent bony bridging across the joints, ultimately leading to complete fusion of the joint (eFig. 19-6.2). X-rays of the neck and the back often show changes that reflect consequences of inflammation at the spinal joints. The earliest changes on lateral view of the spine include shiny vertebral corners, erosions, and squaring of vertebrae. This is followed by bone bridging, or marginal syndesmophytes, beginning from the ends of the vertebrae across the disc space. Complete bony bridging can occur. If most of the vertebrae are bridged, it is called “bamboo” spine. These changes closely resemble the changes in AS (Fig. 19-7). Often in PsA, the syndesmophytes can develop from sites away from the vertebral body. The presence of these “nonmarginal” syndesmophytes is characteristic of PsA (eFig. 19-7.1). The changes described can at occur at any site—cervical and lumbar vertebrae are frequently involved. In the cervical spine, occasionally atlanto-axial subluxation may be present and can lead to serious consequences.
Ultrasound combined with Doppler evaluation, can detect active inflammation in joints and entheses. Ultrasound can also detect erosions before they appear on X-rays especially in the hand joints. It can also be used to guide injections into joints.33
MRI scans along with contrast can detect active inflammation. MRI can also show erosions in the bone even before they are seen on plain X-rays.34 Bone edema which often precedes the development of overt erosions is also detected easily. MRI scans are also very useful in detecting active inflammation in the spine and MRI changes are usually evident much before any abnormality is visualized on X-rays.34 Thus, MRI evaluation is crucial in the diagnosis of early disease, especially affecting the spine.
Course and Prognosis
PsA has a variable course and prognosis. While some patients do well with small number of joints involved, and no significant damage, others progress very quickly, developed marked joint damage and disability.35 Over 10 years of follow-up, 55% of the patients had at least five clinically damaged joints.36 By the time patients present to a PsA clinic, 67% have at least one erosion.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree