div class=”ChapterContextInformation”>
13. Scalp Psoriasis
Keywords
PsoriasisScalp psoriasisTopical corticosteroidsVitamin D analogsPhototherapySystemic treatmentBiologic agentsIntroduction
Psoriasis is a systemic inflammatory disorder affecting approximately 1–3% of the world population. Cutaneous lesions are characterized by well-demarcated, erythematous plaques with silver scale. The etiology is multifactorial, with a clear genetic predisposition and environmental and behavioral factors affecting the course of disease. Psoriasis is a complex immune-mediated disease in which T lymphocytes and dendritic cells play a central role. The scalp is the most common and frequently the first site of disease involvement. The estimated population prevalence of scalp psoriasis in Western Europe is 2%. However, up to 80% of patients report some degree of scalp involvement, and most of them report a negative impact on their quality of life. Scalp psoriasis (SP) treatment is difficult because of the limited access and visibility of the scalp through the hair, making patients nonconscious about treatment. Despite the wide range of available therapies, long-term management has been unsatisfactory, often because of safety concerns about chronic drug use [1–5].
Diagnostic Procedures and Labs Required Before Starting Treatment
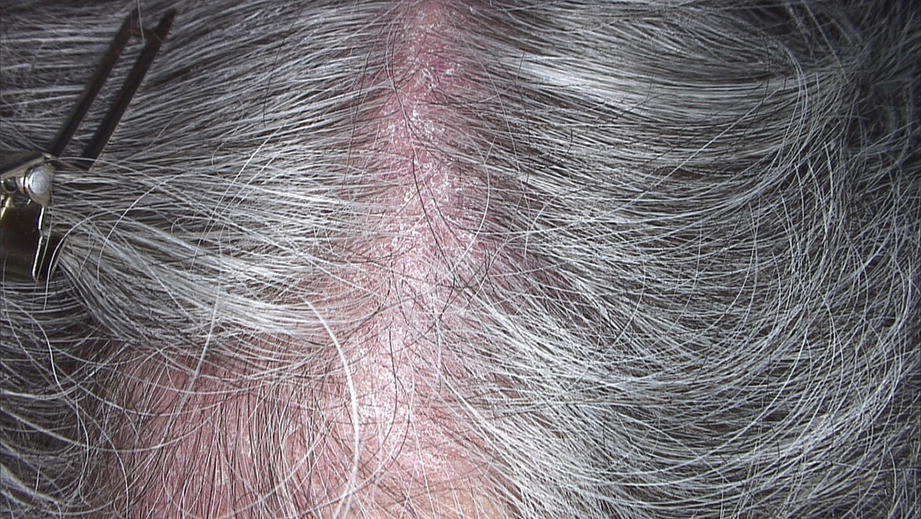
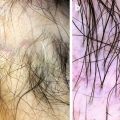
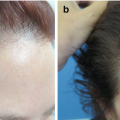
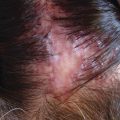
Scalp psoriasis. Erythema and silver white scales
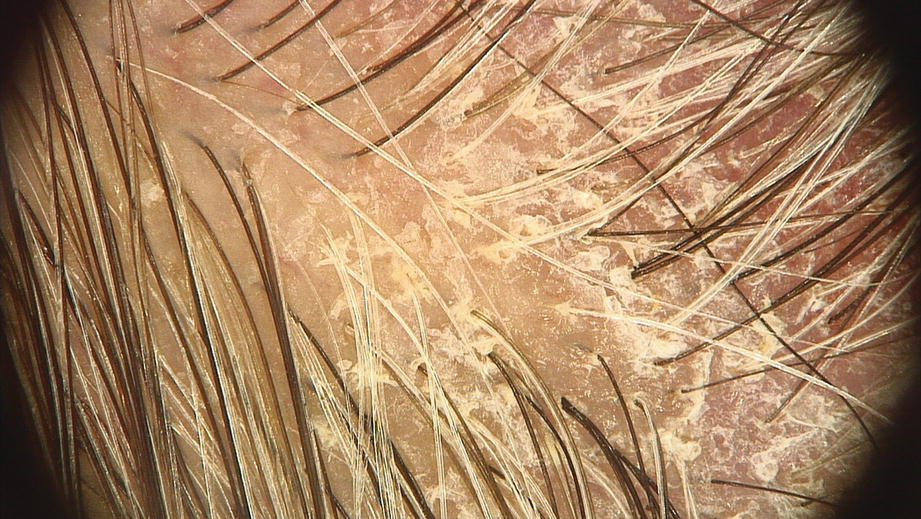
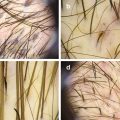
Interfollicular and perifollicular white scales (dermoscopy 20×)
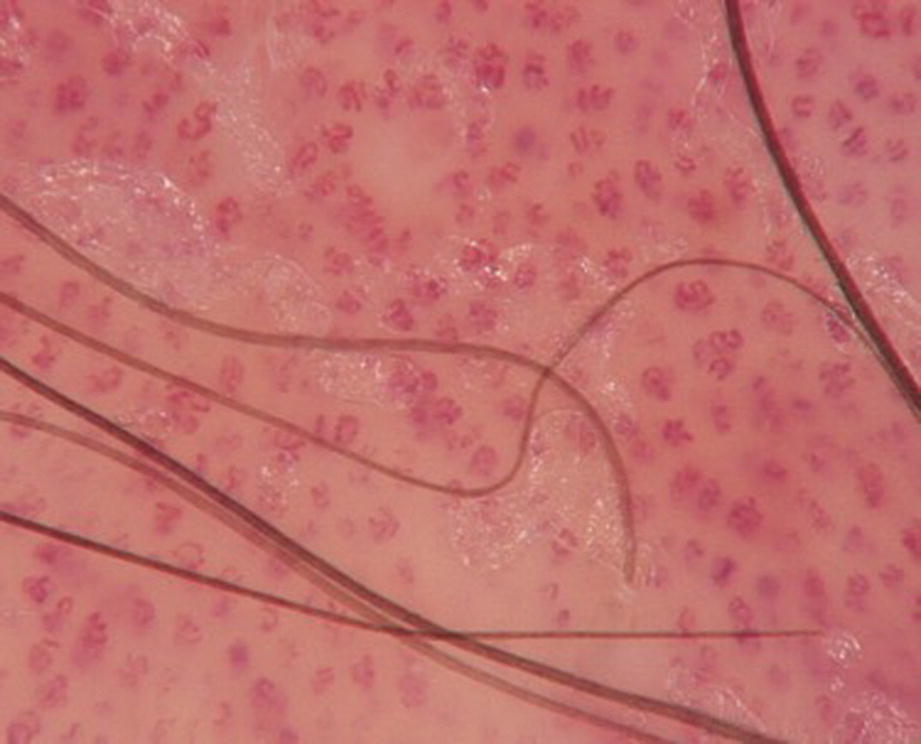
Vascular pattern: red dots and globules (dermoscopy 20×)
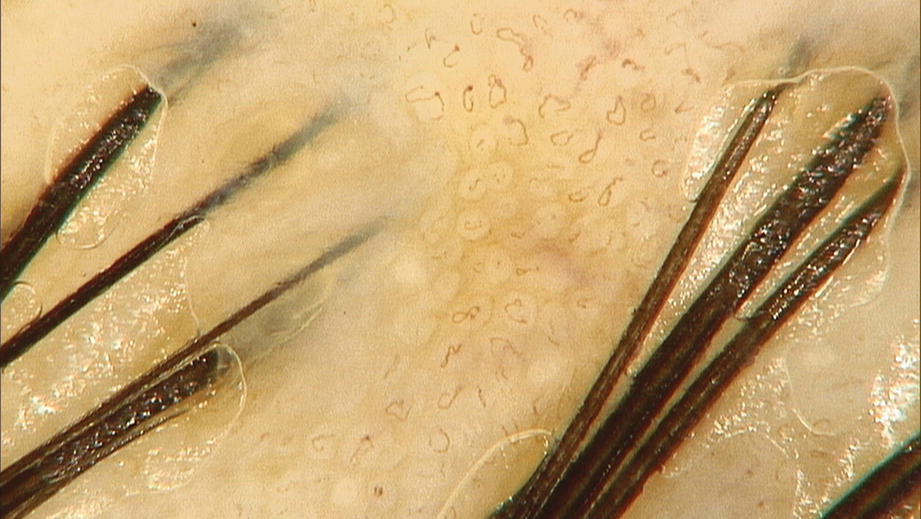
Twisted red loops (dermoscopy 50×)
Treatment Strategy: A General Introduction
Scalp psoriasis treatments
First-line treatments (A) Topical corticosteroids (B) Vitamin D analogs (B.1) Calcipotriene (B.2) Calcitriol (C) Coal tar (D) Topical salicylic acid and other α-hydroxy acids (E) Topical anthralin (F) Topical tazarotene |
Second-line treatments (A) Phototherapy (A.1) Ultraviolet B (UVB) (A.2) Narrowband UVB (A.3) Photochemotherapy (PUVA) (A.4) Excimer laser |
Third-line treatments (A) Systemic treatment (A.1) Methotrexate (A.2) Acitretin (A.3) Cyclosporine (A.4) Apremilast (A.5) Biologic agents (A.5.1) Etanercept (A.5.2) Infliximab (A.5.3) Adalimumab (A.5.4) Ustekinumab (A.5.5) Secukinumab (A.5.6) Ixekizumab (A.5.7) Brodalumab (A.5.8) Guselkumab (A.5.9) Tildrakizumab (A.5.10) Certolizumab pegol (A.5.11) Itolizumab (A.6) Other immunosuppressive agents (A.7) Fumaric acid esters (A.8) Therapies targeting the Th17 pathway (risankizumab and bimekizumab) (A.9) Small molecules (JAK inhibitors) |
First-Line Treatments [12–14]
Topical Corticosteroids
Topical corticosteroids remain the first-line treatment for SP despite the development of newer agents [15]. They have anti-inflammatory, antiproliferative, and immunosuppressive properties affecting the gene transcription . On the scalp, potent corticosteroids in a solution vehicle (fluocinonide 0.05% or clobetasol propionate 0.05%) are frequently indicated. Other vehicles like shampoos, foams, or sprays can also be used. They are the most effective drugs in clearing SP signs and symptoms (inflammation, erythema, scaling, and pruritus). Prolonged continuous use is not recommended due to possible side effects (telangiectasias, hypertrichosis, atrophy, and perioral dermatitis). Prescribe a high or ultra-high topic corticosteroid daily for 4 weeks (occlusive in severe cases) . The authors prefer a lotion or gel versus a shampoo to avoid unnecessary exposure to other body areas. Once clinical improvement occurs, the frequency of application should be reduced [16]. For patients in whom lesions recur quickly, topical corticosteroids can be applied intermittently (two times per week for several weeks or months) to maintain improvement. The combination of betamethasone/salicylic acid is very useful to remove scales [17–20].
Topical Vitamin D Analogs
Topical vitamin D analogs for psoriasis include calcipotriene (calcipotriol), calcitriol, and tacalcitol. Although they are effective as monotherapy for some patients, combination therapy with topical corticosteroids is more effective [15, 16, 21–25].
Calcipotriene (Calcipotriol)
Calcipotriene’s mechanism of action is unclear, having a hypoproliferative effect on keratinocytes and acting as an immunomodulator. Skin irritation is the main adverse event. The combined use of calcipotriene with ultra-potent corticosteroids has demonstrated increased clinical response and tolerance in clinical trials compared with either agent used alone. It is applied in lotion twice per day for 8–12 weeks when used as monotherapy and in gel once per day for 4–8 weeks when used in combination with corticosteroids. Acidic products (salicylic acid shampoo) can inactivate topical calcipotriene, so each must be used daily at different times. Ketoconazole-based shampoo might reduce the irritation, as Malassezia is more abundant in scalp areas irritated by topical calcipotriol treatment [15, 16, 21–26].
Calcitriol
The mechanism of action of calcitriol is thought to be similar to calcipotriene, with the added effect of inhibiting T-cell proliferation and other inflammatory mediators. In a systematic review, calcipotriene and calcitriol were equally effective. It is applied twice per day for 8–12 weeks.
Coal Tar
Coal tar is a mixture of phenols, polycyclic aromatic hydrocarbons, and heterocyclic compounds with anti-inflammatory, anti-itch and keratolytic properties. Tar products are available without a prescription in the form of shampoos, creams, lotions, solutions, foams, ointments, and oils that must be applied daily or two to three times per week for 4–8 weeks. Side effects include skin irritation, sun sensitivity, allergic reactions, and skin discoloration. Its use in pregnancy and breastfeeding is not recommended. Tar shampoos can be helpful when combined with topical corticosteroids or vitamin D analogs. There are no commercially available corticosteroid /tar combinations. A good option for SP is 4–10% LCD (liquor carbonis detergens [a tar distillate] mixed in mineral oil) used daily or two to three times per week for 4–8 weeks. Patients should be warned that tar products have the potential to stain hair, skin, and clothing. It may help to use them at night and wear inexpensive night clothes (old pajamas). Patients may find the odor of tar products unpleasant. For shampoos, be sure the product reaches the scalp. Tar shampoo should be left in place for 5–10 minutes before rinsing it out [27–29].
Topical Salicylic Acid and Other α-Hydroxy Acids
Salicylic acid (SA) and α-hydroxy acids are keratolytic agents that help to shed psoriatic scales and increase drug penetration of topical agents. The mechanism of action of these acids includes exfoliation and stimulation of epidermal renewal. Although the actual benefit of using keratolytic agents in SP is unknown, the authors often prescribe a compound formulation containing 5% salicylic acid in equal parts of petrolatum, almond oil, and lanolin for 4 or 5 days to remove thick scales. After that, a lotion containing salicylic acid alone or in association with a topical steroid can be applied daily or three to four times per week for 4–8 weeks. Once clinical improvement occurs, the frequency of application should be reduced (two times per week for several months). Shampoos containing salicylic acid can be utilized twice a week; instruct the patient not to use for hair but just for the scalp. Salicylic acid inactivates vitamin D analogs so they should not be applied together [30–32].
Topical Anthralin (Dithranol)
Dithranol inhibits keratinocyte hyperproliferation and granulocyte function and has an immunosuppressive effect. The dithranol molecule contains both hydrophilic and lipophilic centers that can be incorporated into detergents to allow easy removal from hair. This property has led to the incorporation of dithranol in an emulsifying oil base (bio-wash oil) or shampoo. In order to minimize irritation, treatment is usually prescribed as a short-contact regimen that is titrated according to patient tolerance. For example, treatment may begin with low concentrations of 0.1% or 0.25% applied for 10–20 min/day, with weekly stepwise increases in duration to reach a total contact time up to 1 hour. Then weekly serial increases in the concentration of anthralin can be performed (0.5%, 1%, and 2%) based on patient tolerance and clinical response. After the desired contact period has elapsed, anthralin should be washed off the treated area, making sure that it does not get in contact with the eyes. The side effects include skin irritation, sun sensitivity, allergic reactions, permanent red-brown stains on clothing, and temporary staining of skin [33–35].
Topical Tazarotene
Tazarotene is a retinoic acid receptor-specific retinoid with demonstrated efficacy in the topical treatment of psoriasis. It downregulates markers of keratinocyte differentiation, keratinocyte proliferation, and inflammation. The 0.1% gel is more effective than 0.05% gel but with a slightly higher rate of local side effects. Apply the gel daily or three times per week every night for 4–8 weeks. Side effects include severe skin irritation, sun sensitivity, and allergic reactions. It can be used in combination with topical corticosteroids or phototherapy to improve the results [36, 37].
Second-Line Treatments
Phototherapy
Ultraviolet (UV) radiation is very effective for the control of psoriatic skin lesions. UV radiation has antiproliferative and anti-inflammatory effects (inducing apoptosis of pathogenic T cells). Treatment of SP with phototherapy (UVA or UVB) or excimer laser is difficult since hair shields the scalp from ultraviolet (UV) radiation. UV combs have been developed for scalp use, and blow dryers may help expose the scalp for excimer laser (308 nm) treatment [38–50].
Third- and Fourth-Line Treatments
Systemic Treatment
A variety of systemic medications are used for psoriasis; they are indicated in patients with more than 5% of body surface area involvement or relapsing disease. Most guidelines recommend these treatments when topical agents fail, and close monitoring for adverse events is suggested [51, 52].
Systemic therapy includes immunosuppressive or immunomodulatory drugs such as methotrexate, cyclosporine, acitretin, apremilast, and biologic agents. Biologic agents are the most effective therapies for moderate-to-severe psoriasis. Although efficacy of systemic treatments for psoriasis is proved, consideration of other factors such as side effects, patient preference, drug availability, and costs also play an important role in treatment selection [49, 50].
Methotrexate
Folic acid antagonist has antiproliferative effects on DNA synthesis in epidermal cells and immunosuppressive effect on activated T cells. It is usually administered in an intermittent low-dose regimen (once a week). Administration can be oral, intravenous, intramuscular, or subcutaneous; the usual dose range is between 7.5 and 25 mg/week. Treatment is usually started at 10–15 mg weekly. Dose can then be escalated every 4–8 weeks depending on tolerance, efficacy, and toxicity. Methotrexate can be used for long-term therapy, but monitoring for bone marrow suppression and hepatotoxicity is necessary. Folic acid 1 mg/day protects against some of the common side effects. Concurrent use of other medications that interfere with folic acid metabolism, such as sulfa antibiotics, can increase the toxicity of methotrexate . Liver biopsy should be considered after a cumulative dose of 3.5–4 g of methotrexate has been administered [53, 54].
Acitretin
Systemic retinoids are utilized for patients with severe psoriasis, including pustular and erythrodermic forms and in patients with HIV-associated psoriasis. The retinoid of choice is acitretin with a dose range of 25–50 mg daily. Acitretin can be used in combination with UVB or PUVA therapy with higher response rates. Monitoring for hypertriglyceridemia and hepatotoxicity is required with retinoid therapy. Common side effects include cheilitis and telogen effluvium, so this drug should be avoided in patients with SP associated with hair loss or androgenetic alopecia. Acitretin is teratogenic; therefore, it is only indicated in men and women of nonreproductive potential. Pregnancy is contraindicated for 3 years after discontinuing the drug [55–57].
Cyclosporine
The T-cell suppressor cyclosporine is effective in severe psoriasis. Usual doses are of 3–5 mg/kg/day orally, and improvement is generally observed within 4 weeks. Close monitoring is required for renal toxicity and hypertension [58, 59].
Apremilast
Apremilast is a phosphodiesterase 4 inhibitor that reduces the production of multiple cytokines involved in the pathogenesis of psoriasis. It is indicated for the treatment of moderate to severe plaque psoriasis in patients who are candidates for phototherapy or systemic therapy. The recommended dose is 30 mg twice/day . Efficacy on SP has been proven by a double-blind clinical trial. When treatment is started, there is a short-term risk of diarrhea (15–20%). Tolerability improves by slowly ramping up the dose in the first 6 days when treatment is initiated. Side effects include nausea, upper respiratory infection, headache , and weight loss. Worsening depression, suicidal thoughts, or other mood changes have also been reported [60, 61].
Biologic Agents
The available biologics for psoriasis have excellent short-term and long-term efficacy and favorable tolerability. They include etanercept, infliximab, adalimumab, ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, and certolizumab pegol. The TNF-alpha inhibitors have the potential to activate latent infections such as tuberculosis and herpes zoster, and the anti-IL-17 has been associated with a slight increased risk of Candida infections [62–65]. Etanercept, infliximab, alefacept, ustekinumab, and secukinumab have been used specifically in some SP studies [66–70]. Severe scalp psoriasis can develop during treatment with TNF alfa inhibitors for inflammatory bowel disorders.
Etanercept
Etanercept is a TNF-alpha inhibitor approved by the FDA for adults with psoriatic arthritis and for patients (>4 years old) with chronic moderate-to-severe plaque psoriasis. Standard dosing for adults is subcutaneous injection of 50 mg twice weekly for the initial 3 months, followed by a 50 mg injection once weekly for maintenance therapy. Standard pediatric dosing is 0.8 mg/kg/weekly, with a maximum dose of 50 mg/week [71, 72].
Infliximab
Infliximab is a TNF-alpha inhibitor indicated in moderate-to-severe plaque psoriasis. It is well tolerated, and its onset of action is faster than other biologic agents. Standard dosing for adults is intravenous infusion of 5 mg/kg at weeks 0, 2, and 6, followed by every 8 weeks thereafter. Infliximab is also an effective treatment for psoriatic arthritis. Studies on psoriasis , inflammatory bowel disease, and rheumatoid arthritis have suggested that the production of antibodies to infliximab may contribute to the loss of response. Anti-infliximab antibodies have been reported to occur in 5–44% of patients with psoriasis [73–75].
Adalimumab
Adalimumab is a humanized monoclonal antibody with activity against TNF-alpha, approved by the FDA for treatment of moderate-to-severe chronic plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Standard dosing for adults is an initial subcutaneous injection of 80 mg followed by 40 mg every other week . Antibodies against adalimumab are reported in 6–50% of patients treated with psoriasis and may reduce response to therapy. Adalimumab is also effective for psoriatic arthritis [76, 77].
Ustekinumab
Ustekinumab is a human monoclonal antibody that targets IL-12 and IL-23 and is indicated for the treatment of adults and children >12 years and older with moderate-to-severe psoriasis who are candidates for phototherapy or systemic therapy. Dosing of ustekinumab is weight-based. Standard dosing for adults ≤100 kg is 45 mg given at weeks 0 and 4 and every 12 weeks thereafter. A 90-mg dose given in the same regimen is recommended for adults over 100 kg. Ustekinumab can also improve psoriatic arthritis. Because of its immunomodulatory mechanism of action, there is concern that this drug may increase the risk for infections, cardiovascular events, and malignancy, but this has not been proved. Anti-ustekinumab antibodies have been reported to occur in 4–6% of patients treated for psoriasis [78–80].
Secukinumab
Secukinumab is an anti-IL-17A monoclonal antibody, effective for moderate-to-severe plaque psoriasis. Standard dosing is 300 mg given subcutaneously once weekly at weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks. Doses of 150 mg are sufficient for some patients. Secukinumab is also effective for psoriatic arthritis [81, 82].
Ixekizumab
Ixekizumab is a humanized monoclonal antibody against IL-17A, effective in the treatment of moderate-to-severe plaque psoriasis in adults. Standard dosing is 160 mg at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12. Subsequently , 80 mg is given every 4 weeks. Ixekizumab is also effective for psoriatic arthritis [83, 84].
Brodalumab
Brodalumab is an anti-IL-17 receptor A monoclonal antibody with high efficacy for psoriasis. It is FDA-approved for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies. Recommended dosing is 210 mg given at weeks 0, 1, and 2 and then every 2 weeks. There are some concerns about the risk for suicidal ideation and completed suicides in treated patients. However, a causal relationship between brodalumab treatment and suicidal ideation and behavior has not been confirmed [85, 86].
Guselkumab
Guselkumab is a human immunoglobulin G1 (IgG1) lambda monoclonal antibody that binds to the p19 subunit of IL-23. IL-39 also contains this p19 subunit. The mechanism of action in psoriasis is thought to involve inhibition of IL-23 signaling. Recommended dosing is 100 mg at weeks 0 and 4 and then every 8 weeks. Guselkumab is being evaluated for psoriatic arthritis [87, 88].
Tildrakizumab
Tildrakizumab is a human immunoglobulin G1 (IgG1) kappa monoclonal antibody that binds to the p19 subunit of IL-23. It is FDA-approved for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy . Recommended dosing is 100 mg given subcutaneously at weeks 0 and 4 and then every 12 weeks [89].
Certolizumab Pegol
Certolizumab pegol is a pegylated humanized antibody Fab fragment with specificity for TNF-alpha. It is FDA-approved for the treatment of adults with moderate-to-severe psoriasis who are candidates for systemic therapy or phototherapy. Standard dosing for certolizumab is 400 mg/week. An optional regimen for patients who weigh ≤90 kg is 400 mg at weeks 0, 2, and 4, followed by 200 mg every other week. A potential advantage of certolizumab pegol is minimal transfer across the placenta; unlike other anti-TNF biologics, certolizumab pegol does not bind the neonatal Fc receptor because it lacks the IgG Fc. It is also effective for psoriatic arthritis [90, 91].
Itolizumab
Itolizumab is a monoclonal antibody against the T-cell co-stimulator CD6. It is a biologic agent that is available for the treatment of psoriasis in India [92].
Other Immunosuppressive Agents
These drugs include hydroxyurea, 6-thioguanine, and azathioprine, which are some alternatives for severe psoriasis when other systemic modalities cannot be used. Oral tacrolimus requires larger studies before it can be considered an accepted psoriasis alternative.
Fumaric Acid Esters
Fumaric acid esters (fumarates) have been used to treat psoriasis in Northern Europe. A systematic review of randomized trials found evidence to support superior efficacy compared with placebo for psoriasis ; however, the quality of the evidence was low. Lymphopenia and progressive multifocal leukoencephalopathy are rare and severe side effects of the use of fumarates [93, 94].
Therapies Targeting the Th17 Pathway
Interleukins (ILs) in the Th17 pathway (IL-23 and IL-17) play a pivotal role in the pathogenesis of psoriasis and have become targets for drug development. These drugs include IL-23/IL-39 inhibitor risankizumab (humanized monoclonal antibody directed against the p19 subunit of IL-23 and IL-39) and bimekizumab (humanized monoclonal antibody targeting IL-17A and IL-17F) [95, 96].
Small Molecules
Other potential therapies include various small molecules that target the interruption of cellular signaling, critical for propagation of the inflammatory response. These drugs include molecules that block Janus kinase (JAK), lipids, a protein kinase C inhibitor, and a selective tyrosine kinase 2 (TYK2) inhibitor [97–102].
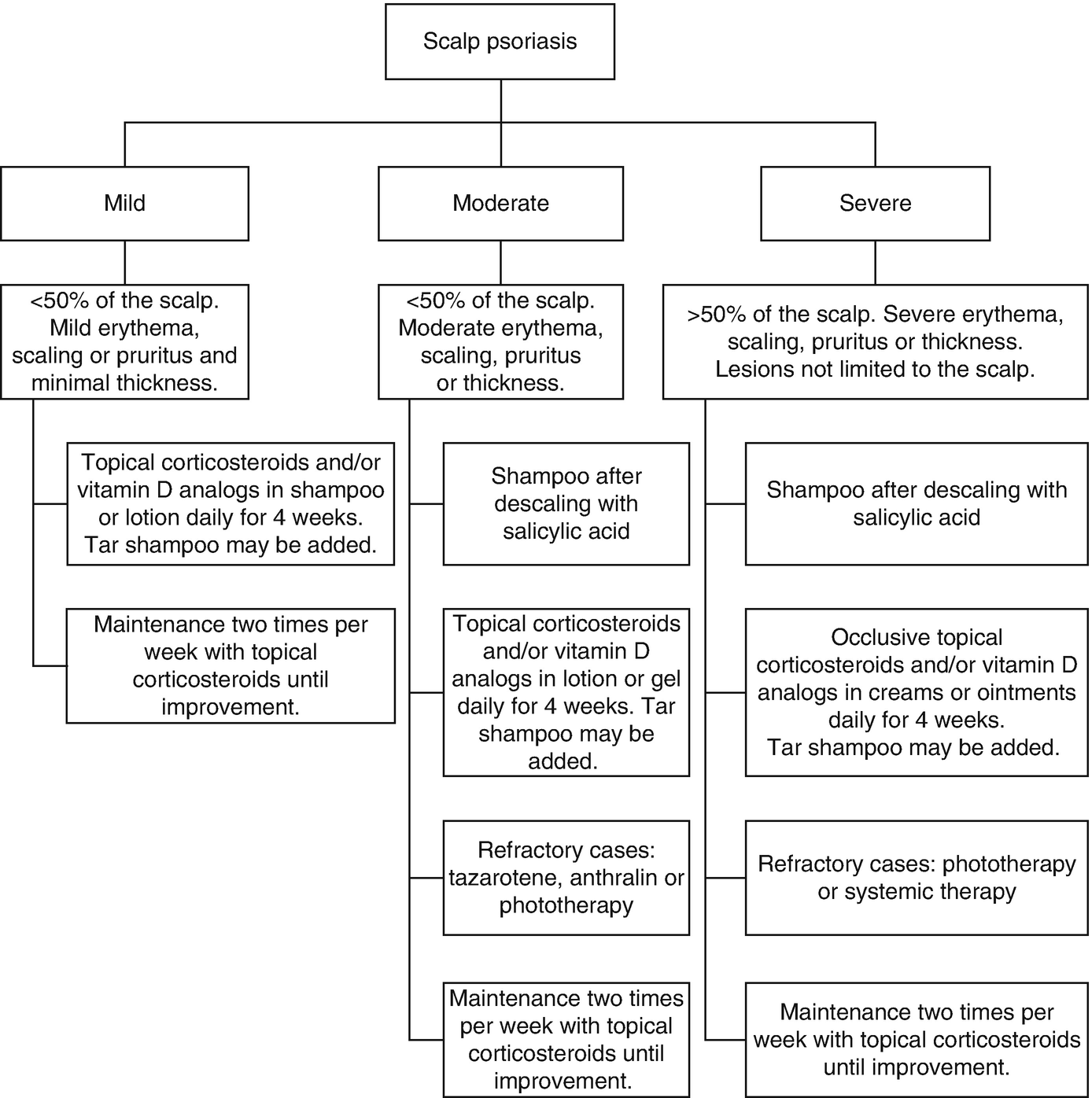
Treatment Selection (Fig. 13.5)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree