Principles and Techniques of Peripheral Nerve Repair, Grafts, and Transfers
Susan E. Mackinnon
Stephen H. Colbert
Injuries to peripheral nerves may be devastating due to the incomplete nature of nerve healing and the possibility of permanent functional impairment. Peripheral nerve injuries require appropriate management to optimize motor and sensory recovery and to minimize pain. The surgeon must accurately identify the injury, determine the primary therapeutic goal, and decide if and when to operate. The management of peripheral nerve injuries has benefited from clinical experience gained in World War II, the evolution of microsurgical technique, improvements in surgical equipment, and the consistently advancing field of neuroscience.
NERVE ANATOMY AND PHYSIOLOGY
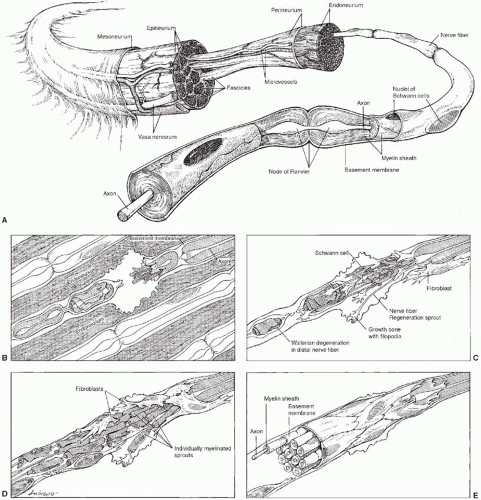
In the normal nerve (Figure 9.1), axons are either unmyelinated or myelinated. Unmyelinated axons are ensheathed by a single Schwann cell-derived double basement membrane, whereas myelinated axons are surrounded by a multilaminated, laminin-rich, myelin sheath with stacks of individual Schwann cells along the length of the axon. Individual nerve fibers are surrounded by the thin collagen of the endoneurium. Fibers destined for a specific anatomic location are grouped together in fascicles surrounded by the perineurium. The connective tissue that surrounds the peripheral nerve is the epineurium. A thin layer of loose areolar tissue, the mesoneurium, connects the epineurium to the surrounding structures and allows for the uninhibited excursion of nerves within the extremities. Regional arteries and veins supply the vasa nervorum, longitudinal vessels running along the epineurium that communicate with intraneural vessels running within the perineurium and the endoneurium. Bidirectional axonal transport within the nerve fiber is responsible for structural support of the nerve and delivery of neurotransmitters and trophic factors. In the normal nerve, the intrinsic blood supply is substantial, allowing mobilization and elevation of nerves over a long distance (bipedicle width:length ratio of 64:1).
NERVE INJURY
Traumatized peripheral nerves are characterized by specific changes both proximal and distal to the site of injury. Proximally, axons retract a variable distance depending on the degree of injury and after a brief period of quiescence elongate as a hydra-like regenerating unit in which a single parent axon gives rise to multiple daughter axons. In myelinated nerves, axons sprout at unsheathed gaps known as the nodes of Ranvier and progress to their sensory or motor targets. Observations and elegant studies by Cajal, Sunderland, Lundborg, Brushart, Mackinnon, and others have shown that regenerating axons do not always take a direct course but do preferentially target their appropriate end-organ receptors.1,2,3,4,5 Once a functional synapse is made, the remaining daughter axons degenerate, or are “pruned back.” In the distal nerve segment, Schwann cells, fibroblasts, myocytes, and injured axons express a host of neurotrophic factors, including glial and brain-derived neurotrophic factors at discrete concentrations and time points as the degrading neural elements are phagocytosed in a process termed Wallerian degeneration. Neurotrophism, which literally means food for nerves, is the ability of neurotrophins secreted in an autocrine or paracrine fashion to enhance the elongation and maturation of nerve fibers. Schwann cells assume a pro-regenerative phenotype instrumental in remyelinating and guiding regenerating axons to their appropriate targets along residual endoneurial tubes. The orderly arrangement of these Schwann cells along the endoneurium forms the bands of Bungner. Functional recovery depends on the number of motor fibers correctly matched with motor endplates and the number of sensory fibers correctly matched with sensory receptors.
Experimental studies show that regenerating fibers can demonstrate both tissue and end-organ specificity.5 This process is called neurotropism. The preference of a nerve fiber to grow toward a nerve versus other tissue depends on a critical gap across which the fiber responds to the influences of the distal nerve. Current research suggests that the expression of various Schwann cell and myelin-associated glycoproteins may facilitate or impede the regeneration of damaged axons to their correct targets.6
CLASSIFYING NERVE INJURIES
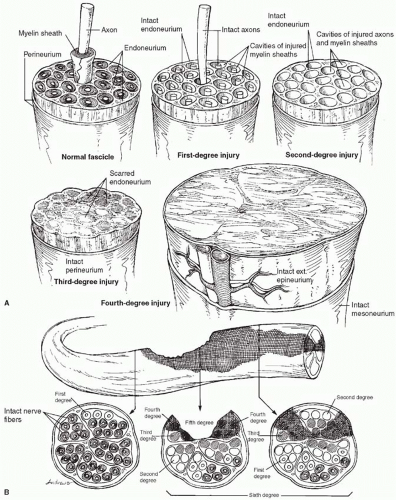
The classification of nerve injuries, originally proposed by Seddon in 19437 and Sunderland in 1951,8 was subsequently expanded by Mackinnon9 to include a sixth category representing a mixed injury pattern (Figure 9.2). The level and degree of injury are important in determining treatment. First-, second-, and third-degree injuries have the potential for recovery and for the most part do not require surgical intervention. A first-degree injury recovers function quickly (within 3 months). A second-degree injury recovers slowly (1 inch per month) but completely, whereas recovery after third-degree injuries is slow and incomplete. Fourth- and fifth-degree injuries will not recover without surgical intervention. A sixth-degree injury shows a variable recovery.
First-degree injury (neurapraxia). A localized conduction block is produced that may result in segmental demyelination. Because the axons are not injured, regeneration is not required and remyelination and complete recovery occur within 12 to 16 weeks.
Second-degree injury (axonotmesis). Axonal injury occurs and the distal segment undergoes Wallerian degeneration. Proximal nerve fibers will regenerate at a rate of 1 inch per month. By definition, the connective tissue layers are uninjured. Recovery will be complete. The progress of regeneration can be followed by the advancing Tinel sign.
Third-degree injury. Wallerian degeneration is combined with some fibrosis of the endoneurium. Recovery will be incomplete because scar within the endoneurium may block or cause mismatching of regenerating fibers with the appropriate end organs. Surgery is indicated if the lesion localizes to a known area of entrapment where nerve regeneration is delayed. The recovery is uniformly better than that
seen with a repair or graft unless it is associated with severe causalgia.
seen with a repair or graft unless it is associated with severe causalgia.
Fourth-degree injury. The nerve is in continuity but with complete scar block resulting from injury to the endoneurium and perineurium. Regeneration will not occur unless the block is excised and the nerve is repaired or grafted.
Fifth-degree injury (neurotmesis). The nerve is completely divided and must be repaired before any regeneration can occur.
Sixth-degree injury. This represents a combination of any of the previous five levels of injury. Because of the longitudinal nature of crushing injuries, different levels of nerve injury can be seen at various locations along the nerve. This is the most challenging nerve injury for the surgeon as some fascicles will need to be protected and not “downgraded,” whereas others will require surgical reconstruction.
Proper clinical assessment is paramount to development of a treatment plan. The extent of motor nerve injury is
determined by an evaluation of weakness, loss of motion, and atrophy. The extent of sensory nerve injury is determined by moving and static two-point discrimination, which are measurements of innervation density and the number of fibers innervating sensory end organs. Light moving touch, for example, evaluates the innervation of large A-β fibers and can be quickly screened with the valid and reliable “Ten Test.”10 Patients rank the quality of sensation in the affected digit compared with that in the normal contralateral digit using a scale from 0 to 10. Vibration instruments and Semmes-Weinstein monofilaments are also used as threshold tests to evaluate the performance level of nerve fibers and are more useful in evaluating chronic compressive neuropathies. Testing is also performed after nerve repair to assess the quality of nerve repair, determine the need for revision, and monitor recovery.
determined by an evaluation of weakness, loss of motion, and atrophy. The extent of sensory nerve injury is determined by moving and static two-point discrimination, which are measurements of innervation density and the number of fibers innervating sensory end organs. Light moving touch, for example, evaluates the innervation of large A-β fibers and can be quickly screened with the valid and reliable “Ten Test.”10 Patients rank the quality of sensation in the affected digit compared with that in the normal contralateral digit using a scale from 0 to 10. Vibration instruments and Semmes-Weinstein monofilaments are also used as threshold tests to evaluate the performance level of nerve fibers and are more useful in evaluating chronic compressive neuropathies. Testing is also performed after nerve repair to assess the quality of nerve repair, determine the need for revision, and monitor recovery.
Sharp nerve injuries are treated with repair or reconstruction in a timely fashion, generally with minimal delay unless required to achieve a healthy wound bed. Closed injuries are treated expectantly up to 12 weeks to allow for first-, second-, and third-degree injuries to show signs of recovery. Recovery is assessed with serial physical examinations and electrodiagnostic nerve studies at 6 and 12 weeks. This allows for the accurate assessment of the degree of injury and appropriate subsequent treatment plan. Fibrillations on electromyography (EMG) indicate axonal injury and will be present around 6 weeks postinjury (second-, third-, fourth-, and fifth-degree injuries). By contrast, the presence of motor unit potentials (MUPs) does not occur until about 12 weeks postinjury. MUPs are present in second- and third- but not fourth- and fifth-degree injuries. The presence of MUPs on EMG is a contraindication to surgery except for a simple decompression at distal sites of compression. MUPs indicate collateral sprouting of intact nerve fibers. Nascent units will occur later as actual injured axons regenerate to motor targets. MUPs and nascent units are not present in fourth- and fifth-degree injuries.
PRINCIPLES OF NERVE REPAIR
Basic principles of nerve repair include the use of meticulous microsurgical techniques with adequate magnification, microsurgical instruments, and sutures. When the clinical and surgical conditions allow, a primary nerve repair is performed in a tension-free manner. To facilitate the repair, the injured segments of the nerve can be mobilized or, in the case of the ulnar nerve at the elbow, transposed, to obtain length. Intrinsically, peripheral nerves do afford a limited degree of excursion. This property of intrinsic redundancy or elasticity gives the peripheral nerves a horizontal or spiral banded appearance called the bands of Fontana.11 The bands of Fontana are created by laxity in nerve fibers. Thus, their presence in an injured nerve will let the surgeon know that nerve fibers (first-, second-, or third-degree injury) are present. This finding is helpful in evaluation of in-continuity nerve injuries. These bands disappear when the nerve is compressed or stretched. Extremes in the range of motion of joints in the vicinity of the repair and facilitation of an end-to-end repair with postural positioning of the extremity are discouraged. If a tension-free repair cannot be achieved, an interposition nerve graft is preferable with the limb in a neutral position. In an effort to match sensory and motor modalities and to optimize the specificity of nerve regeneration, a grouped fascicular repair should be performed whenever the internal topography of the nerve is segregated into motor, sensory, or regional components. Otherwise an epineural repair is performed. Postoperative motor and sensory reeducation maximizes the surgical result.
FASCICULAR IDENTIFICATION
The object of peripheral nerve repair is to restore the continuity of motor and sensory fascicles in the proximal segment with the corresponding fascicles in the distal segment. The internal organization of nerves is distinct even in the proximal extremity, although nerves in the proximal extremity are monofascicular. There is considerable plexus formation between the fascicles, which decreases in the distal extremity. As nerves progress distally, they become polyfascicular and the fascicles are further differentiated into motor or sensory components.12,13 In the proximal segment of the nerve, motor fibers are distinguished from sensory fibers by knowledge of the internal topography, intraoperative stimulation, or “neurolysis with the eyes.”14 Using this technique, the distal stump of the injured nerve is dissected to discern motor from sensory fascicles. These fascicles are then visually traced back to the level of injury.
Knowledge of the usual internal topography of the peripheral nerves can direct proper alignment of fascicles at the time of nerve repair. For example, the fascicles of the ulnar nerve in the mid- and distal forearm are divided into a dorsal sensory group, a volar sensory group, and a motor group. In the mid-forearm, the motor group is positioned between the ulnar dorsal sensory group and the radial volar sensory group (Figure 9.3). The dorsal sensory group separates from the main ulnar nerve approximately 8 to 10 cm proximal to the wrist. The motor group remains ulnar to the volar sensory group until the Guyon canal, at which time it passes dorsally and radially to become the deep motor branch to the intrinsic muscles. The motor group is two-thirds the size of the sensory group at this level. The median nerve topography is more complex because it contains more fascicles. In the forearm, the anterior interosseous nerve is situated in the radial or posterior aspect of the median nerve as a distinct group. The distal internal topography of the median nerve approximates the distal anatomy; the motor fascicles to the thenar muscles are on the radial side and the sensory fibers to the third web space are on the ulnar side. Our web site, nerveinjury.wustl.edu, details the internal topography of the various nerves.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree