I. AIRWAY
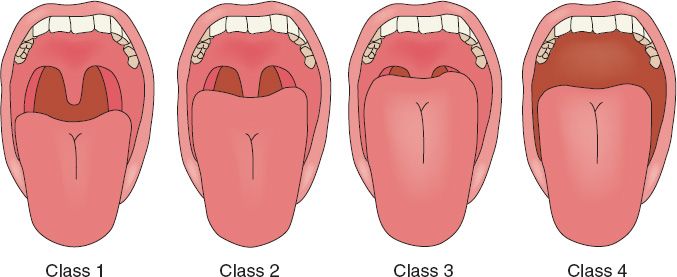
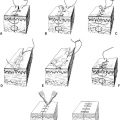
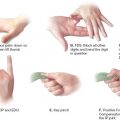
A. Mallampati scores (Fig. 59-1): A high Mallampati score (either 3 or 4) is associated with more difficult mask ventilation and intubation.
1. Class I: Full visibility of tonsils, uvula, and soft palate
2. Class II: Visibility of hard and soft palates, upper portions of tonsils, and uvula
3. Class III: Soft and hard palates, as well as base of uvula, are visible
4. Class IV: Hard palate is the only visible structure
B. The LEMON method of airway assessment is a useful screening tool. Patients who meet multiple LEMON criteria should be referred for preoperative anesthesia consultation.
1. L = Look externally (for beard/mustache, facial trauma, macroglossia, micrognathia).
2. E = Evaluate the 3-3-2 rule.
a. Mouth opening <3 finger breadths (the patient’s fingers) with normal dentition quality.
b. Hyoid-mentum distance less than 3 finger breadths.
c. Thyroid cartilage–hyoid bone <2 finger breadths.
3. M = Mallampati score of 3 or 4.
4. O = Obstruction (from large tonsils, peritonsillar abscess, trauma, macroglossia).
5. N = Neck mobility (cervical extension and flexion).
C. Other patients to refer to preoperative anesthesia for airway issues include
1. Patients with known obstructive sleep apnea.
2. Patients with a history of difficult airway.
3. Patients with other barriers to intubation (such as a halo).
4. Super-morbid obesity.
II. RISK STRATIFICATION AND PROPHYLAXIS FOR ENDOCARDITIS
A. In general, invasive procedures performed through surgically scrubbed skin are not likely to produce clinically relevant bacteremia.
1. If the planned procedure involves skin, subcutaneous tissue, muscle, or bone, your patient does not need endocarditis prophylaxis.
2. If the planned procedure involves an epithelial-lined tract, your patient requires risk stratification and prophylaxis.
B. Level of endocarditis risk
1. High risk category
a. Prosthetic cardiac valves.
b. Previous bacterial endocarditis.
c. History of cyanotic congenital heart disease (e.g., tetralogy of Fallot, transposition of great arteries).
d. Surgically constructed pulmonary shunts or conduits.
2. Moderate risk category
a. Other congenital cardiac malformations not listed above.
b. Hypertrophic cardiomyopathy.
c. Known mitral valve dysfunction.
d. Mitral value prolapse with regurgitation or thickened leaflets.
3. Low risk category: Everybody else
______________
*Denotes common in-service examination topics
Figure 59-1. Mallampati classification.
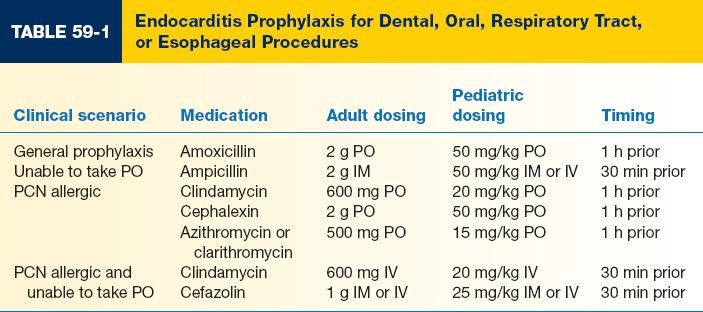
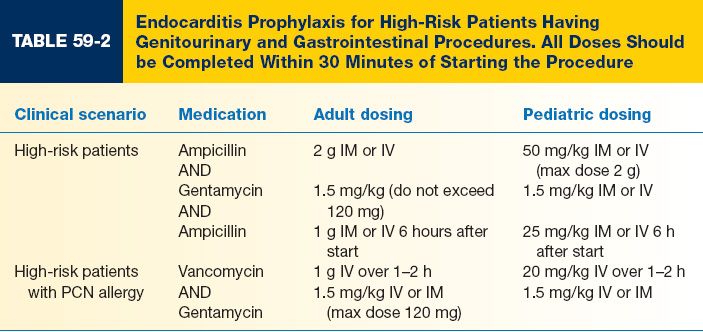
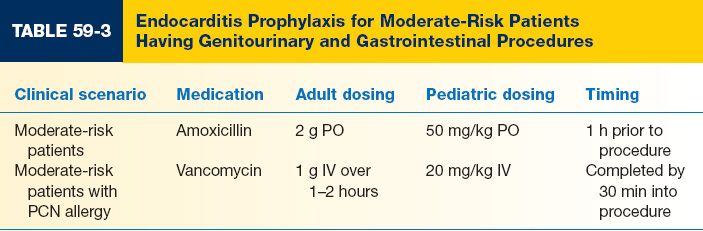
C. Appropriate prophylaxis stratified by patient and procedure type is shown in Tables 59-1, 59-2, and 59-3.
III. AMERICAN HEART ASSOCIATION GUIDELINES FOR PERIOPERATIVE β-BLOCKADE
A. Presence of one or more of the 2009 AHA/ACC criteria is associated with increased risk for a cardiac event.
1. Patients with drug-eluting stents who are not permitted to continue aspirin perioperatively.
2. Unstable angina
3. Myocardial infarction within the past month.
4. Congestive heart failure.
5. Severe valvular disease.
6. Significant cardiac arrhythmias.
7. Exercise tolerance less than 4 METs (inability to climb one flight of stairs).
B. Preoperative evaluation
1. If the patient is already on a β-blocker for a cardiovascular indication (angina, arrhythmia, hypertension), plan to continue this medication in the perioperative period.
2. Consider starting higher risk patients on β-blockade at least 1 week prior to surgery, or refer them back to their primary care provider (PCP) to address this issue.
3. Higher risk patients include
a. Vascular surgery patients with coronary artery disease.
b. Vascular surgery patients with multiple cardiac risk factors.
c. Patients with cardiac ischemia present on preoperative testing.
d. Patients having high-risk procedures for cardiac events.
4. The utility of β-blockade is unknown for lower-risk patients, including
a. Patients undergoing low or intermediate risk surgery.
b. Vascular surgery patients without known coronary artery disease.
C. Postoperative management
1. Continue β-blockers in patients receiving them preoperatively for cardiovascular indications.
2. Watch for hypotension and bradycardia.
IV. MALIGNANT HYPERTHERMIA
A. Malignant hyperthermia
1. Life-threatening reaction to certain anesthetic agents, including volatile anesthetics and succinylcholine.
2. Exposure to these drugs causes an uncontrolled increase in skeletal muscle metabolism.
3. The disorder will exhaust all available oxygen and exceed the body’s ability to excrete carbon dioxide.
4. A potentially fatal increase in body temperature is also seen.
5. Increased temperature is a late sign.
6. Initial signs can be increased in end tidal carbon dioxide, muscle rigidity, and tachycardia/arrhythmia.
7. Left unchecked, circulatory collapse and death will occur.
8. Inherited in an autosomal dominant fashion.
9. *Appropriate treatment includes cooling treatment, IV dantrolene, and discontinuation of offending agents, in addition to cardiovascular support.
10. Patients with a personal or family history of malignant hyperthermia should be referred to anesthesia preoperatively.
A. Venous thromboembolism (VTE) includes deep venous thrombosis and pulmonary embolism
1. Major source of morbidity and mortality among hospitalized patients.
2. Considered potentially preventable through use of sequential compression devices and, in some cases, chemoprophylaxis like heparin or low-molecular weight heparin.
B. VTE risk stratification and prophylaxis for inpatient surgery
1. 2005 Caprini Risk Assessment Model (Table 59-4) has been validated to predict 60-day VTE risk in plastic surgery patients.
2. If NO chemoprophylaxis is given, expected 60-day VTE rates include
a. Caprini scores of 3–4 = 0.6%
b. Caprini scores of 5–6 = 1.3%
c. Caprini scores of 7–8= 2.7%
d. Caprini scores of >8 = 11.3%
3. For inpatients with Caprini scores of >7, weight-based prophylactic dose enoxaparin given during the inpatient stay can decrease observed 60-day VTE rate by 50%.
a. When started 6 to 8 hours after surgery, a 0.7% increased rate of reoperative hematoma can be expected.
b. This can be balanced against the risk reduction for potentially fatal VTE events.
4. Unless a contraindication is present, all patients should have sequential compression device (SCDs) while in the hospital.
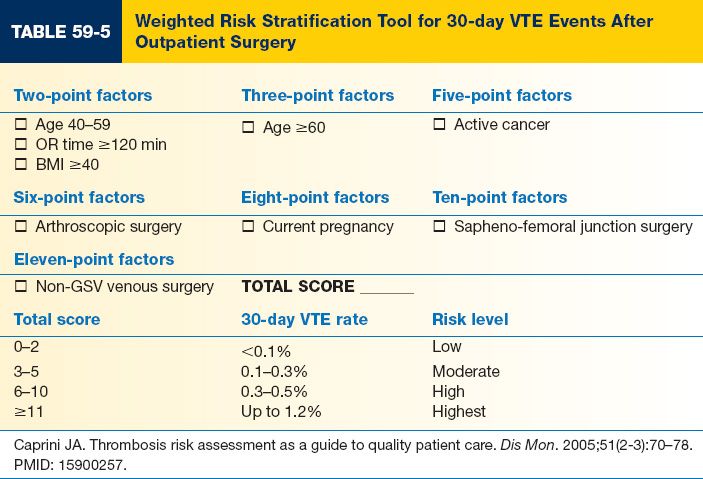
C. VTE risk stratification and prophylaxis for outpatient surgery (Table 59-5)
1. Outpatient surgery is generally considered to be low risk for VTE.
2. Recent risk stratification models have shown that a distinct, high-risk subgroup exists within the generally low-risk outpatient surgery population.
3. The risk stratification model shown below can predict 30-day VTE risk and identify both low- and high-risk patients.
4. No data is available for VTE prevention among outpatients
a. The role of chemoprophylaxis remains unknown.
b. Unless a contraindication is present, all patients having surgery under general anesthesia or IV sedation should have SCDs placed.
ACKNOWLEDGMENTS
Thanks to Norah Naughton, MD, for her critical appraisal of this chapter.
PEARLS
1. “First do no harm.” Assess each surgical candidate for their peri operative risk and treat / prophylaxis accordingly. Anesthetic, endocarditis, cardiac, and VTE risks should be specifically considered.
2. The most overlooked risk factor for VTE is a positive family history.
QUESTIONS YOU WILL BE ASKED
1. Which patient characteristics increase the risk for VTE?
Many factors are known to increase risk for perioperative VTE. Major factors include cancer, central venous catheters, and a personal or family history of VTE.
2. What options exist for prophylaxis against perioperative VTE?
The most important decision-making tool for prophylaxis is appropriate risk stratification. Once risk has been quantified, appropriate prophylaxis may include SCDs, early ambulation, and/or chemoprophylaxis. Risk factor modification is also important in the preoperative setting.
3. What is the appropriate treatment for patients suspected of having malignant hyperthermia?
Appropriate treatment for malignant hyperthermia includes removing the offending agent, administration of dantrolene, and cardiovascular support as necessary.
Recommended Readings
American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Society of Echocardiography, American Society of Nuclear Cardiology, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54(22):e13–e118. PMID: 19926002.
Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2–3): 70–78. PMID: 15900257.
Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation. 1997;96(1):358–366. PMID: 9236458
Pannucci CJ, Dreszer G, Wachtman CF, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128(5): 1093–1103. PMID: 22030491.
< div class='tao-gold-member'>