Hennedige AA, Quaba AA, Al-Nikib K. Plast Reconstruct Surg 2008; 121: 1173–80.
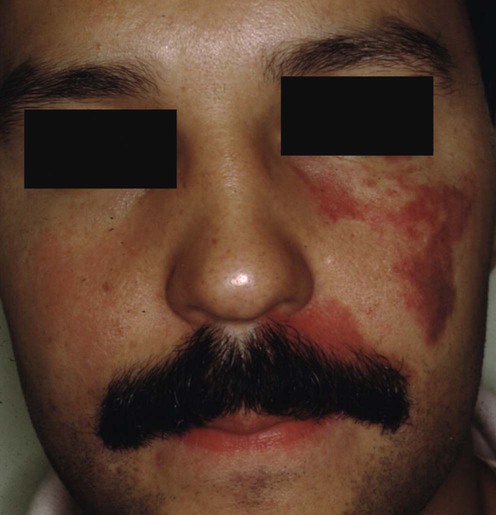
Port wine stain (‘nevus flammeus’)
Specific investigations
Sturge–Weber syndrome and dermatomal facial port wine stains: incidence, association with glaucoma, and pulsed tunable dye laser treatment effectiveness.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




