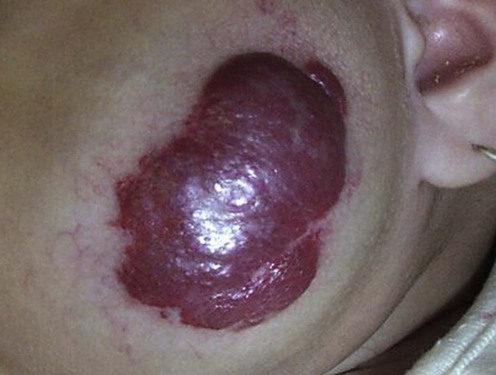
Hemangiomas
Specific investigations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




