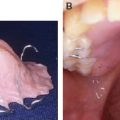
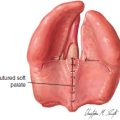
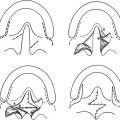
75 ○ The variability in both the inclusion criteria and the differences in terminology that surround Pierre Robin sequence have made actual incidence figures difficult to determine. ○ Surgery candidates with Pierre Robin sequence range from neonates to young adults. The indications for surgery vary with the age of the child, from urgent surgery to alleviate airway obstruction because of a hypoplastic mandible during infancy, to elective orthognathic surgery for adolescents with occlusal and aesthetic concerns. ○ The preoperative evaluation of surgery candidates must be tailored to the underlying issues. ○ Surgical management of airway obstruction in Pierre Robin sequence is largely through one of three approaches: tracheostomy, tongue-lip adhesion, or mandibular distraction osteogenesis. ○ It is occasionally necessary to consider surgery for mandibular lengthening in a child 6 months of age or older. The triad of a hypoplastic mandible, glossoptosis, and airway obstruction has been widely credited to the French “stomatologist” Pierre Robin, based on his report of these findings published in 1923.1 Robin’s2 detailed description of the disorder and his subsequent contributions have led to the eponymous characterization of this triad as Pierre Robin sequence, although it is clear that others had described similar associations before Robin.3–6 Notably, Robin’s initial description of the disorder did not include an association with cleft palate. However, Pierre Robin sequence is frequently associated with cleft palate, with a cleft being present in the majority of cases, ranging from 60% to 90% in most series.7–10 In describing this disorder, the term sequence is most often employed in its name and description, as opposed to syndrome, since the disorder is not believed to be a genetic syndrome, at least in the classic sense. Pierre Robin sequence is frequently associated with other disorders, most commonly Stickler syndrome (high-grade myopia, visual and hearing problems, and a flattened midface and flattened nasal bridge), which occurs in up to one third of all cases.5,11–13 In addition, Pierre Robin sequence can be associated with 22q11.2 deletion syndrome, fetal alcohol syndrome, and Treacher Collins syndrome14,15 (Table 75-1). It is critically important that treating physicians understand these associated syndromes, since appropriate screening and treatment must be implemented to minimize morbidity and optimize care. Table 75-1 Syndromes Associated With Pierre Robin Sequence Pierre Robin sequence has historically been associated with significant morbidity and mortality, most directly related to airway obstruction and its associated problems. Mortality rates, ranging from 30% to 60%, have been reported in the past.1,2,8,16–18 Although Robin described the anomaly as micrognathia, others have described it as retrognathia, or retrodisplacement of the mandible.5 The terminology may vary, but the mandible is hypoplastic, and this contributes significantly to airway obstruction.5,8,19 It is of critical importance to recognize that the tongue’s position in three-dimensional space is defined architecturally by the mandible, since nearly all of the tongue’s muscular attachments are directly to the mandible. When the mandible is hypoplastic, it is deficient in the sagittal plane, and the tongue is of necessity displaced posteriorly. The position of the posterior pharyngeal wall and spinal column cannot change; so, as the tongue is displaced posteriorly, the airway lumen is reduced in size and airway obstruction results directly from the posterior displacement of the tongue. Glossoptosis, described by Robin as a retropositioning of the tongue base both backward and downward toward the glottis, obstructs the airway at the level of the base of the tongue. In glossoptosis, the base of the tongue is drawn downward to cover the epiglottis during inspiration and acts as a ball valve, exacerbating difficulty with ventilation.6,16 Douglas16 developed a model that demonstrated this effect, with the negative inspiratory pressures drawing the base of the tongue down to cover the airway. Glossoptosis is a major cause of impaired ventilation in many patients with Pierre Robin sequence. However, in some patients when Pierre Robin sequence is associated with a cleft palate, the tongue may actually undergo a “displacement” upward into the nasal cavity as the mouth closes. The tip of the tongue comes to lie above the palate in the nasal cavity, with the frenulum lying against the anterior edge of the U-shaped cleft.5,20,21 In these patients, the tongue undergoes displacement upward into the nasal cavity, and it is unlikely that glossoptosis, with its ball valve effect, occurs with the tongue in this superior position. However, Routledge20 commented that he thinks these cases have particularly severe obstruction, because both the oral and nasal airways are completely occupied by the tongue. With contemporary management, the morbidity and mortality from Pierre Robin sequence has improved. Perhaps the largest impact on the improved survival of these neonates and infants has been the early recognition of the disorder, sometimes discovered even prenatally with ultrasonography, which leads to the appropriate level of care for these complex children. This early recognition of Pierre Robin sequence, the concomitant focus on diagnosis and management, the advent and rapid development of the field of neonatology, the development of multispecialty team management, and the refinement of surgical techniques have all contributed to a significant improvement in outcomes for these children. In contemporary management, the morbidity and mortality is more frequently caused by the associated pathology present with associated syndromes than that related directly to Pierre Robin sequence.9 That being said, the surgical management of Pierre Robin sequence is challenging and demands the respect and the completely focused attention of those involved in the care of these patients to ensure a favorable outcome. The critical issues in the surgical management of Pierre Robin sequence distill down to the issues in most surgical procedures: These questions are made much more complex in Pierre Robin sequence because of the critical nature of the airway disorder, the small size of the anatomic structures, and the impact of time and growth on the airway in the infant. The variability in both the inclusion criteria and the differences in terminology that surround Pierre Robin sequence have made actual incidence figures difficult to determine. However, considering that the overall incidence of cleft palate (not associated with cleft lip) is approximately 1 in 2000 births, an approximate incidence of Pierre Robin sequence of between 1 in 7500 to 1 in 10,000 births can be calculated. This incidence has been corroborated by at least two studies, one of these a regional catchment area study in England and the other from a major birthing hospital in the United States.22,23 There is a spectrum of severity in children with Pierre Robin sequence. Those affected can vary in regard to their micrognathia, glossoptosis, and airway obstruction. The majority of patients with Pierre Robin sequence will not require surgery for airway obstruction during infancy. Many neonates and infants at the milder end of the spectrum can be adequately and appropriately treated through prone positioning only. When the child is in the prone position, gravity can be a sufficient force to allow the tongue to fall forward off of the posterior pharyngeal wall to ameliorate the glossoptosis, leading to adequate ventilation. Approximately half of all Pierre Robin patients will be adequately and appropriately managed by prone positioning alone.13,23 However, there are several caveats that must be considered in recommending prone positioning as the intervention for a child with Pierre Robin sequence.24 First, the airway must be stable in the prone position. Second, there must be appropriate home nursing or support services available. Third, the parents must be specifically educated and must be able to comprehend the technique and critical nature of positioning and caring for the child. They must be able to provide care for the child given these considerations. Finally, prone positioning is not without risk, and appropriate precautions are necessary. In view of the American Academy of Pediatrics’ Back to Sleep campaign25,26 and the dangers of prone positioning during infancy, any infant positioned prone for sleep should be placed on an apnea-bradycardia monitor. This is even more crucial in a child with potential breathing problems, such as a child with Pierre Robin sequence; therefore the routine use of an apnea-bradycardia monitor in this population with compromised ventilation is strongly advised, and many view this as essential. The severity of airway obstruction in Pierre Robin was traditionally characterized by the clinical signs of obstruction: snoring, discoloration of the lips, and difficulties in feeding and weight gain. These clinical signs have now been augmented by more sophisticated techniques, including pulse oximetry and multichannel polysomnography (PSG).18 Many times, clinical decisions are made on a health care provider’s observations that a child has desaturation as determined by pulse oximetry. Although this can provide some insight into the severity of the problem in Pierre Robin sequence, multichannel PSG provides a broad spectrum of detailed information for review and analysis.18 The comparison of PSGs performed at two or more time intervals can provide trend data. Significantly, PSG can also be used to assess whether a given intervention is effective in improving the child’s ventilation—in this case, characterizing whether the prone positioning is effective in mitigating the airway obstruction. Unlike PSG in adults, normative data are not as well established in children, and the value of an isolated PSG, or of a specific piece of data from a PSG, is more difficult to interpret than in an adult.27 Nonetheless, PSG can be a powerful tool in characterizing patients with Pierre Robin sequence, specifically to assess whether an intervention is effective.18 As the severity of the airway obstruction increases within the spectrum of Pierre Robin sequence, the micrognathia, glossoptosis, and airway obstruction frequently have additional associations which have a deleterious impact on the infant: feeding difficulties, oropharngyo-esophageal dysmotility, gastroesophageal reflux, aspiration and pneumonitis/pneumonia. These problems can be, and often are, interrelated. The airway obstruction leads to an increased work of breathing, increasing caloric requirements, and increased caloric expenditure just to meet the metabolic needs of breathing; and, this often occurs in an infant who may already be nutritionally compromised because of difficulty with feeding. Many infants with Pierre Robin sequence have feeding difficulties and dysmotility problems.9,18 Airway obstruction leads to increased negative intrathoracic pressures with breathing, as the child struggles to obtain adequate air movement. This not only leads to the obvious clinical finding of sternal and rib retraction in these infants, but also the less obvious, but perhaps the more morbid, association of gastroesophageal reflux.13,18,28 In addition, gasping for breath can fill the stomach with air, increasing the probability of gastroesophageal reflux. Gastroesophageal reflux has been shown to cause obstructive sleep apnea.29 Gastroesophageal reflux, in turn, can also be associated with aspiration, pneumonitis, or pneumonia, exacerbating an already compromised pulmonary status.30 Treatment of the esophageal reflux through positioning, with the head of the bed elevated and thickening feedings, can improve the pulmonary status and child’s overall progress.18 Within the spectrum of Pierre Robin sequence, as the severity of the disorder increases, the need for surgical intervention to manage the airway obstruction becomes necessary. Multispecialty assessment and management is essential. Typically this should include a neonatologist (age appropriate), a pediatric pulmonologist, a pediatric otolaryngologist, a pediatric anesthesiologist and a plastic surgeon with experience in craniofacial surgery. A critical consideration in the decision regarding surgical management is the recognition that the clinical scenario is a four-dimensional problem, with time and growth figuring prominently in the child’s progress. Growth is occurring, and consequently, pulmonary dynamics are changing. The mandible is growing, and with growth, clinical improvement of the obstruction frequently occurs. Further, there exists a school of thought within the management of Pierre Robin sequence that the mandible undergoes “catch-up” growth following birth. Some think that in many infants the mandible grows even faster than would be the case with “normal” growth, and after 1 or 2 years the mandible will essentially be “normal.” The data regarding whether “catch-up” growth occurs are both conflicting and contentious.5 There are studies that indicate that mandibular “catch-up” does occur, and others that suggest that while some growth does occur, the mandible remains essentially hypoplastic.5,19,31 In one of the most detailed and comprehensive studies, Figueroa et al19 determined that the mandible in Pierre Robin patients was hypoplastic at birth and did demonstrate faster growth than control groups of both isolated cleft populations without Pierre Robin and a normal standards group. Figueroa demonstrated that the airway tripled in size during this early growth of infancy; however, the mandible still remained hypoplastic compared with both the cleft non-PRS population and normal controls.19 The variability in the growth of the mandible that occurs during infancy may reflect the diversity of the population grouped together as Pierre Robin sequence patients. One thing is certain: In many infants with Pierre Robin sequence, mandibular growth does occur and airway obstruction, and its downstream consequences, frequently improve if the child can be adequately supported during the first 6 months of life. This is likely a result of the considerations regarding Pouseille’s law of laminar flow—that any change in airway diameter has a very significant impact on flow dynamics. Although the infant’s airway does not strictly conform to the principles of laminar flow (and air is not a noncompressible fluid), Poiseuille’s law predicts that flow will be proportional to the diameter of the airway raised to the fourth power, that is, doubling airway size will lead to 16 times the flow, and halving the airway diameter will lead to #### of the flow. Small changes can be predicted to have a large impact. These changes seen with growth must be considered as the team of physicians and the surgeon consider the choice of surgical interventions. Patients with Pierre Robin sequence who are candidates for surgery cross a large spectrum, from neonates to young adults. The indications for surgery vary with the age of the child, ranging from the urgent need for surgery to alleviate airway obstruction because of a hypoplastic mandible during infancy, to elective orthognathic surgery for adolescents with occlusal and aesthetic concerns. In many cases, the timing of surgical intervention is dictated directly by the clinical scenario, and surgery is not elective. A not uncommon scenario is a neonate with Pierre Robin sequence who is noted to be in extremis after delivery and is intubated rapidly after birth. In this case, if the neonatologists and consultants concur that the airway is a life-threatening concern, it may be necessary to decide on a surgical management paradigm without extubation and further airway evaluation. Typically, these consultants will include a neonatologist, a pulmonary medicine specialist, an otolaryngologist, and a plastic surgeon. If the child has associated findings, such as a congenital cardiac anomaly, then additional input may be necessary from other specialists. A second more common scenario is a neonate who develops airway obstruction, fatigue, and failure to thrive within the first few weeks after birth.5 Although the infant may initially be noted to be “doing well” in the perinatal period, any child with a small mandible should be followed closely. Many of these patients will respond to prone positioning while on an apnea-bradycardia monitor. Additional steps in care include removing everything from the crib or bed other than a sheet, including all bumpers and other objects. Prone positioning is not recommended for any child without appropriate monitoring. Multichannel PSG is obtained with the child in the prone position to confirm that ventilation is improved and the pulmonary obstruction is resolved. If the child does not respond to prone positioning or the response is marginal and a significant degree of obstruction remains, surgery is indicated. A third scenario in which surgery may be indicated is before palatal closure. When the patient reaches the age for palatal closure, if there are persistent signs and symptoms of obstruction and the mandible remains hypoplastic, a PSG can be obtained before palatal closure. Although there are no clear data on this issue, closure of a large palatal cleft may potentially exacerbate findings of obstruction that are present preoperatively. If the PSG performed before palatal closure indicates substantive signs of obstruction and if the mandible has remained hypoplastic, the child may be considered for mandibular distraction osteogenesis. The other two surgical modalities (tracheostomy or tongue-lip adhesion) are not considered at this age and stage. Mandibular distraction may also be indicated at this stage or during early childhood to allow removal of a tracheostomy. Finally, an infant with mandibular hypoplasia may survive infancy and tolerate palatal closure but have persistent mandibular hypoplasia into childhood or adolescence. This may cause persistent breathing irregularities, malocclusion, or significant aesthetic concerns. These patients may be candidates for surgical treatment with conventional orthognathic surgery or with mandibular distraction osteogenesis. The variation in the spectrum of patients who are candidates for surgery is great, ranging from a newborn child with obstructed ventilation to an adult with malocclusion and/or aesthetic concerns. The preoperative evaluation must be tailored to the underlying issues. In a neonate with a hypoplastic mandible who has been urgently intubated, preoperative evaluation consists of appropriate newborn screening and evaluation by consultants from plastic surgery, otolaryngology, neonatology, pediatric pulmonary medicine, and anesthesiology. Genetics evaluation will also be beneficial at this time. In addition to the commonly associated syndromes, there may be occult genetic disorders that are not evident at the time of birth. The child obviously falls into the surgical management paradigm under these unusual circumstances: An experienced pediatric anesthesiologist is essential, as is support from a pediatric otolaryngologist. A neonate with Pierre Robin sequence and airway obstruction is a technically challenging and difficult clinical scenario, with virtually no physiologic margin for error. This surgery should not be undertaken except by those with experience in these types of cases. In a slightly older infant (1 week to 6 months) with a hypoplastic mandible and obstructive sleep apnea/airway obstruction, evaluation by the previously mentioned specialists, as well as the patient’s clinical course, must be considered and weighed carefully. Assessment of the clinical course should be supplemented by the use of nasopharyngoscopy/bronchoscopy and multichannel PSG. Nasopharyngoscopy/bronchoscopy is used to exclude any additional bronchopulmonary tract pathologic conditions that could alter the surgical approach. Multichannel PSG can facilitate characterization of the severity of the disorder, as well as any trend over time. In addition, it can also characterize the adequacy of the treatment intervention. Although data exist that indicate that long-term airway obstruction can be detrimental with an apnea-hypopnea index (AHI) of 5 or greater, particularly in adults, in infants there are mitigating factors to consider.32 One of the main mitigating factors, as discussed previously, is the consideration of growth. In the Pierre Robin population we are working in the realm of a rapidly changing environment during infancy and factors that tend to improve with growth and time. Therefore the team needs to consider the totality of the child’s progress before deciding for or against surgery. Interpretation of the multichannel PSG should include consideration of all parameters (hypoxia, carbon dioxide retention, duration of obstructive episodes, and other factors), but certainly an important parameter is the AHI. In patients with an AHI of 15 or greater, strong consideration should be given to surgical intervention. If the AHI is less than 15, continued observation with continued hospitalization and a repeat PSG study in 1 week may be considered. A clinical trend of improvement with weight gain and overall progress should be followed with continued observation; however, a deterioration in PSG parameters and/or lack of clinical progress suggests that surgical intervention is necessary. If the infant grows and continues with weight gain and improvement in other clinical parameters beyond 4 to 6 months of age, the need for mandibular distraction osteogenesis is less common to correct airway obstruction or obstructive sleep apnea. Indications for mandibular distraction surgery beyond 4 months of age may include consideration of obvious mandibular hypoplasia with an occlusal disorder, or continued borderline airway obstruction that persists while the child is approaching timing for palatal closure. Surgical closure of the palate narrows the aperture of the airway at the level of the palatal plane and may potentially tip a marginally compromised child into obstruction. Surgery for airway obstruction may occasionally need to be considered to allow palatal closure. In these older patients beyond the neonatal period, the same specialists should be involved in the preoperative evaluative process: anesthesiology, pediatric pulmonology, pediatric otolaryngology, plastic surgery, and genetics. The surgeon and entire team must give careful consideration to all events that may potentially occur during the procedure, coordinating a plan to handle adverse events occurring during surgery. This procedure may be performed in infants as small as 2 kg, and the margin of reserve in these infants is extremely limited. First and foremost, the team must be aware of the airway at all times. Intubation can be performed either transnasally or orally, either by direct laryngoscopy or more commonly by using fiberoptic technique. The endotracheal tube can be taped to the upper lip; however, care needs to be taken during taping and prepping to be certain that good adhesion to the skin is both obtained and retained. The neonate/infant’s trachea is short, and the potential for extubation is a constant possibility. The short trachea can lead to extubation during this event, which can be a major problem in these patients. If this occurs, a “crash” reintubation should be avoided. The surgeon and anesthesiologist must recognize that ventilation can be restored by placement of an oral airway and mask ventilation, followed by reintubation. It must be understood that the anesthesiologist will violate the surgical field to provide effective mask ventilation with an oral airway. This should be expected and planned for so that it is not a surprise to anyone if and when it occurs. The airway must be protected. The child can then be prepared and draped again and the procedure can continue. Appropriate plans must also be made for the conclusion of the surgical procedure. Will the child remain intubated? This may often be necessary, since swelling in the neck can have a potentially significant impact on the airway and ventilation postoperatively. If the infant remains intubated for 48 to 72 hours after the procedure, a return to the operating room for extubation may be the optimal plan. The controlled environment, with ideal conditions for reintubation (bed height, lighting, suction), as well as the availability of fiberoptic equipment and maximal support, provides the best approach for managing these transitions in patients with limited reserve. Surgical management of airway obstruction in patients with Pierre Robin sequence is largely through one of three approaches: tracheostomy, tongue-lip adhesion, or mandibular distraction osteogenesis. Each of these surgical procedures has several considerations that are unique to the clinical setting of an infant with airway obstruction. The goal of the surgeon and team is to select the procedure that allows effective and safe relief of airway obstruction with minimal morbidity and mortality and that ideally provides a durable resolution of the airway obstruction. Tracheostomy has been advanced as providing a “safe and secure” airway. Certainly in the case of a hospitalized patient who is watched and monitored, tracheostomy does provide a safe and secure airway. The technique does have several positive aspects: it permits ventilator support, it allows pulmonary toilet, and it deceases airway resistance. However, tracheostomy in a neonate or an infant is a challenging surgical procedure, and there are several considerations that limit a tracheostomy’s potential to reach the goal of being a safe and secure airway. First, the surgical procedure is performed on a very small and pliable airway, and the procedure has the potential complications of pneumomediastinum or pneumothorax, bleeding, and tracheal stenosis. Second, a tracheostomy in an infant is susceptible to plugging or dislodgement. If either of these events occur, the infant is incapable of responding and rectifying the problem, with potentially catastrophic consequences. This problem can and does occur with tracheostomy during infancy. Third, a tracheostomy in a child with Pierre Robin sequence frequently remains in place for 2 or 3 years. During this time, there can be an adverse impact on upper respiratory hygiene. In addition, a tracheostomy has a high cost, both financially and in terms of the burden of care for parents during this time. Finally, the presence of a tracheostomy may impede speech development for the time that it is present. There are many problems that can and do occur in practice with pediatric tracheostomy, as evidenced by the complication rates in large clinical series. Wetmore et al,33 reviewing 450 pediatric patients who underwent tracheotomy over a 10-year period, reported that there was a 19% incidence of complications within the first postoperative week, and a 58% incidence of late complications, including a tracheotomy-related mortality of 0.5% and a nontracheotomy-related mortality of 22%. Other reports of tracheostomy in children have also reported significant problems with the outcomes of the procedure.34 Tracheostomy is an effective technique for the alleviation of airway obstruction, but it does have some substantial additional considerations that must be considered by the treatment team such as plugging/obstruction of the tracheostomy in a child that cannot clear the problem, duration of tracheostomy, and the physical and financial cost. Tongue-lip adhesion is a surgical method of physically attaching the tongue to the lip, and is based on the principle that tethering the tongue anteriorly will prevent the tongue from falling backward and occluding the airway. The procedure was first described by Douglas9 but has undergone evolution and refinement by many surgeons, notably Randall5 and Routledge.20 Additional revisions were made by Larossa et al, and the following technique is based on a further evolution of their described technique.35 Ideally, the child undergoes nasotracheal intubation, often through a fiberoptic approach. Following the secure attachment of the endotracheal tube, a posteriorly based flap is elevated through an inverted U-shaped incision made on the ventral surface of the tongue. A spinal needle is passed through this incision in the tongue while being observed by a fiberoptic scope positioned in the nasopharynx. Thus the position of the puncture of the spinal needle through the base of the tongue is specifically controlled so that the puncture is in the midline and located in the base of the tongue above the epiglottis (Fig. 75-1, A). A 30-gauge wire lasso is threaded through the spinal needle cannula and used to capture the 2-0 braided nonresorbable suture, which is attached to a polyethylene button. This button also has a 3-0 silk suture placed to retrieve the button after the core stitch is released. The spinal needle is removed, and the wire lasso draws the 2-0 braided nonresorbable suture through the tongue. This positions the button on the base of the tongue and is confirmed with the fiberoptic scope. Confirmation of anterior displacement of the tongue with clearing of the airway is confirmed before the scope is removed.
Pierre Robin Sequence: Surgical Management
Robert J. Havlik
KEY POINTS
Syndrome
Percentage
Stickler syndrome
34
Velocardiofacial syndrome/22q11.2
11
Fetal alcohol syndrome
10
Provisionally unique pattern syndromes
10
Treacher Collins syndrome
5
PATIENT POPULATION
TIMING OF SURGERY
PREOPERATIVE EVALUATION
PREOPERATIVE PLANNING
SURGICAL PROCEDURES
Tracheostomy
Tongue-Lip Adhesion
Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine