The Physiology of Wound Healing: Injury Through Maturation
Keywords
• Wound healing • Skin physiology • Soft tissue injury • Coagulation cascade • Fibroplasias
Injury
The initiation of healing starts with the creation of a wound. A wound is defined as an injury to the body that typically involves laceration or breaking of a membrane and damage to the underlying tissues.1 Injury can occur from any number of mechanical or thermal forces that lead to disruption of the skin and damage to the connective tissue and vasculature. Bleeding ensues along with exposure of collagen, endothelium, and intravascular and extravascular proteins. This environment serves as a stimulus for hemostasis.
Hemostasis
Vasoconstriction
Contraction of the smooth muscle within the endothelium is the first response to vessel injury. Reflexive vasoconstriction occurs before activation of platelets and coagulation. The endothelium of damaged vessels produces its own vasoconstrictor, endothelin. Other mediators for vasoconstriction are derived from circulating catecholamines (epinephrine), the sympathetic nervous system (norepinephrine), and the release of prostaglandins from injured cells.2 Coagulation and platelet activation contribute additional stimuli for vasoconstriction through the following mediators: bradykinin, fibrinopeptides, serotonin, and thromboxane A2.
Coagulation Cascade
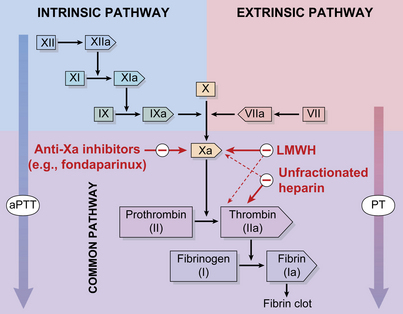
The coagulation cascade is made up of two converging pathways: extrinsic and intrinsic. The extrinsic coagulation pathway is an essential pathway for normal thrombus formation. It is initiated by exposed tissue factor on the subendothelial surface.2 Tissue factor binds to factor VII and leads to the subsequent activation of factors IX and X. The intrinsic pathway is not essential to coagulation. As suggested by name, all components of the pathway are intrinsic to the circulating plasma.3 Initiation of the intrinsic pathway is through the autoactivation of factor XII. Factor XII has the unique ability to change shape in the presence of negatively charged surfaces.4 Factor XII, in its active form, is a stimulus for the activation of factors XI, IX, VIII, and X. Although each pathway has a distinct trigger, both lead to the activation of factor X and the production of thrombin. Thrombin serves two important roles in clot formation: a catalyst for the conversion of fibrinogen to fibrin and an initiator for platelet activation (Fig. 1).5
Platelets Adherence, Aggregation, and Degranulation
Platelets are the first cells to respond in wound healing. Activated platelets contribute to hemostasis through the process of adherence, aggregation, and degranulation. The presence of platelets at the site of injury is stimulated by exposed collagen and thrombin. Collagen within the subendothelial matrix comes in contact with blood flow, leading to the adhesion of circulating platelets. Platelet adherence is achieved through interactions between platelet glycoproteins VI and collagen. Additional interactions occur between platelet glycoprotein Ib-V-IX complex and collagen-bound von Willebrand’s factor. Platelet integrins play a supportive role in the adherence of platelets to collagen, von Willebrand’s factor, fibrinogen, and other platelets.5
As mentioned above, tissue factor activates the extrinsic coagulation pathway leading to the production of thrombin. Thrombin is an independent initiator of platelet activation. Thrombin interacts with a receptor on the platelet surface (Par1) and leads to the release of ADP, serotonin, and thromboxane A2.5 These substances enhance platelet aggregation. Thromboxane A2 and serotonin also act as potent mediators of vasoconstriction.3 Platelet aggregation in the environment of the fibrin matrix forms a clot.
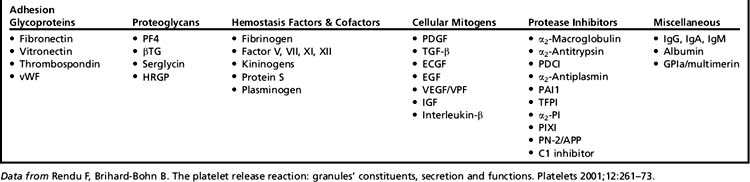
Thrombus prevents ongoing bleeding, establishes a protective barrier, and provides a reservoir for substances released by platelet degranulation. Degranulation involves the release of numerous cytokines, growth factors, and matrix proteins stored within platelet alpha granules. These substances promote a variety of cellular and extracellular mechanisms important to hemostasis as well as several other stages of wound healing: matrix deposition, chemotaxis, cell proliferation, angiogenesis, and remodeling (Table 1).3,6
Inflammation
Achievement of hemostasis leads to the immediate onset of inflammation. Inflammation is evident through the physical signs of erythema, heat, edema, and pain. On a cellular level, inflammation represents vessel dilation, increased vascular permeability, and leukocyte recruitment to the site of injury. Two leukocyte populations sequentially dominate the inflammatory events of wound healing: neutrophils and macrophages. Both provide the critical function of wound debridement, whereas the latter also promotes ongoing cellular recruitment and activation necessary for subsequent steps in wound healing (Fig. 2).
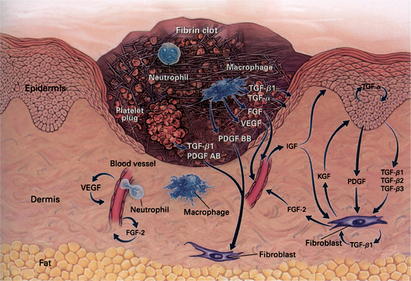
Fig. 2 Inflammatory phase day 3.
(From Singer AJ, Clark RAF. Mechanisms of disease: Cutaneous wound healing. N Engl J Med 1999;341:739; with permission. Copyright © 1999, Massachusetts Medical Society.)
Vasodilation and Increased Permeability
The establishment of vasoconstriction for hemostasis lasts only minutes before several factors stimulate the reverse response of vasodilation. Vasodilation is mediated by the presence of kinins, histamine, prostaglandins, and leukotrienes.2 Vascular dilation increases blood flow to the wound, resulting in the characteristic inflammatory signs of erythema and heat. Increased flow also hastens the delivery of circulating cells and mediators to the site of injury. As vessels dilate, gaps form between the endothelial cells, increasing vascular permeability. Many of the same mediators of vasodilation (prostaglandins and histamine) also stimulate increased vascular permeability. Vasodilation in conjunction with increased permeability allows the transport of intravascular fluid, protein, and cellular components into the extravascular space. The extravasation fluid and migration of cells result in wound edema.
Leukocyte Migration and Chemotaxis
Although plasma passively leaks between endothelial gaps and proteins adhere to the wound matrix, leukocytes undergo the active process of diapedesis to enter the wound. Selectins provide weak adherence between leukocytes and the endothelium of capillaries. Stronger bonds are created between leukocytes, surface integrins, and intercellular adhesion molecules on the endothelial surface.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree