Facial paralysis is a rare but severe condition in the pediatric population. Impaired facial movement has multiple causes and varied presentations, therefore individualized treatment plans are essential for optimal results. Advances in facial reanimation over the past 4 decades have given rise to new treatments designed to restore balance and function in pediatric patients with facial paralysis. This article provides a comprehensive review of pediatric facial rehabilitation and describes a zone-based approach to assessment and treatment of impaired facial movement.
Key points
- •
The main goals of facial reanimation are to restore symmetry, balance, resting tone, and movement to the face, and to decrease hyperfunction from aberrant regeneration.
- •
Special considerations in the pediatric population include the ability to understand and participate in rehabilitation, small-caliber vessels and nerves, and concerns about long-term outcomes with continued craniofacial growth.
- •
An individualized treatment plan is developed for each patient. The paralyzed face is assessed and treated by zone.
- •
Free muscle transfer for smile reanimation in children is safe and has superior results compared with adults. It should be considered as first-line therapy for children with lack of meaningful smile.
- •
Treatments directed at decreasing synkinesis, including botulinum toxin, physical therapy, and surgery, are critical in the overall treatment of children with facial paralysis.
Historical perspective
Harii and colleagues first described gracilis free muscle transfer with microvascular anastomosis for facial rehabilitation in 1976. Free muscle transfer has since become the gold standard for dynamic smile restoration in the adult population. The treatment of impaired facial movement in children is less defined. Investigators including Ueda and colleagues, Terzis and colleagues, Zuker and colleagues, and Hadlock and colleagues have focused specifically on the pediatric population to provide treatment algorithms for children. Advances in facial reanimation over the past 4 decades have given rise to new treatments designed to restore balance and function in pediatric patients with facial paralysis.
Historical perspective
Harii and colleagues first described gracilis free muscle transfer with microvascular anastomosis for facial rehabilitation in 1976. Free muscle transfer has since become the gold standard for dynamic smile restoration in the adult population. The treatment of impaired facial movement in children is less defined. Investigators including Ueda and colleagues, Terzis and colleagues, Zuker and colleagues, and Hadlock and colleagues have focused specifically on the pediatric population to provide treatment algorithms for children. Advances in facial reanimation over the past 4 decades have given rise to new treatments designed to restore balance and function in pediatric patients with facial paralysis.
Introduction
Facial palsy in the pediatric population is a rare condition, with an incidence of 21.1 per 100,000 per year for children less than 15 years old. Facial paralysis has variable presentations, ranging from complete hypofunction to hyperfuction and mixed presentations. Injury to the facial nerve can have severe consequences, including physical deformity, ocular complications, nasal valve collapse, inability to express emotion, oral incompetence, and speech difficulty. The treatment of pediatric facial paralysis is especially challenging because of additional psychosocial concerns, the impact of future growth and potential disfigurement, anatomic considerations, and complex parent decision making.
Pediatric facial paralysis is classified as congenital or acquired. Congenital facial paralysis is uncommon and has multiple causes including birth trauma, Moebius syndrome, unilateral lower lip paralysis, hemifacial microsomia, Goldenhar-Gorlin syndrome, CHARGE (Coloboma, Heart defects, Atresia choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, and Ear abnormalities and/or hearing loss) association, Arnold-Chiari malformation, and syringobulbia. Physical examination and the presence of synkinesis can help distinguish between traumatic and developmental deficits. Acquired facial paralysis in the pediatric population may be caused by infection, Bell’s palsy, neoplasm, or trauma. The most common cause of pediatric facial paralysis is debated in the literature. Although Bell’s palsy occurs less frequently in children compared with adults, multiple studies have found that Bell’s palsy accounts for most pediatric facial paralysis, occurring in 40% to 50% of cases. Other studies have found an identifiable cause in most patients, citing that fewer than 20% of children were diagnosed with Bell’s palsy. Both Grundfast and colleagues and Evans and colleagues found that infectious causes, most frequently otitis media, and trauma were the most common causes of facial nerve paralysis in children.
Treatment goals
When facial nerve injury occurs, the primary goal is to reestablish continuity of the facial nerve via direct neurorrhaphy or cable graft. Facial reanimation procedures are performed when reestablishing the nerve is not possible or when reestablishment of the nerve leads to unacceptable results. The main goals of facial reanimation procedures and adjuvant therapies are to restore symmetry, balance, resting tone, and movement to the face, and to decrease hyperfunction from aberrant regeneration. The specific treatment goals vary with each patient.
Preoperative planning and preparation
Preoperative planning begins with a thorough history to determine the cause of facial paralysis and the likelihood of spontaneous recovery. Cognitive evaluation is also important in children, because physical therapy and muscle training are often components of facial reanimation. All patients undergo zonal facial assessment, including documentation of resting position and movement on eFACE ( Fig. 1 ), photography of 7 standard facial expressions, videography, and spontaneous smile assay. The clinician then develops an individualized treatment plan. If the treatment algorithm includes surgery, medical clearance is required.
There are special considerations in the pediatric population. Although children as young as 2 years old have successfully undergone free tissue transfer for smile restoration, waiting until at least 5 or 6 years of age, around the time the child is school aged and becomes self-aware, is preferred. Delaying major procedures until this age provides time for growth of nerves and vessels, whose small caliber may lead to free flap failure, and allows children to be mature enough to understand and participate in their own care. There is a theoretic concern that surgery may disrupt continued craniofacial growth and lead to disfigurement; however, this has not been established in human studies. In addition, some investigators have found poorer results after long-term follow-up of free flaps for facial reanimation, raising the concern that free flaps in childhood might not function adequately into adulthood. Multiple investigators have refuted these results, reporting excellent aesthetic and functional long-term outcomes following free flap smile reanimation.
Procedural approach to zone-based facial reanimation surgery
Ocular Rehabilitation
After facial nerve injury, ocular protection to prevent dryness, corneal abrasion, and irreversible blindness is paramount. In all children with incomplete eye closure, an aggressive eye care regimen, including artificial tears during the day and ophthalmic ointment with eyelid patching at night, should be established immediately.
Static procedures
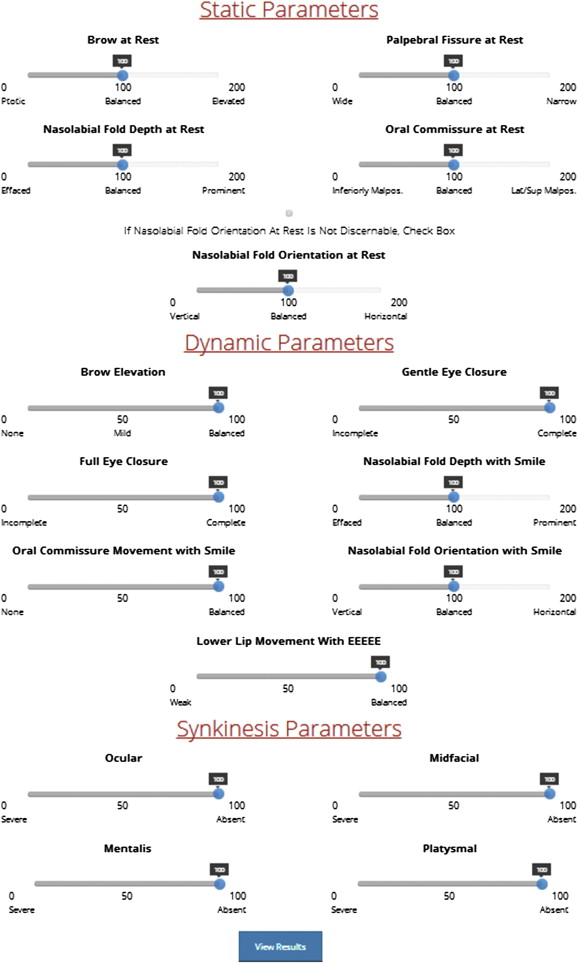
Ocular reanimation may involve static and/or dynamic techniques. Pediatric patients with lagophthalmos are candidates for static eyelid procedures to passively assist in upper eyelid closure, including eyelid weights or palpebral springs. Platinum weights are preferred in adults and children because of their thinner profile, decreased tendency for capsule formation, and lower rates of extrusion. In cooperative children, the procedure may be performed under local anesthesia. For younger children or those who require multiple procedures for facial reanimation, the eyelid weight is placed under general anesthesia. The supratarsal crease is marked before surgery. The patient is placed in a supine position. An incision is made in the crease, and dissection is performed through the orbicularis oculi to the tarsal plate. The implant is centered between the midpupillary line and the medial limbus and sutured in 3 places to the tarsal plate with 6-0 clear nylon sutures ( Fig. 2 ). Eyelid weights can easily be removed during a brief office procedure if facial paralysis resolves.
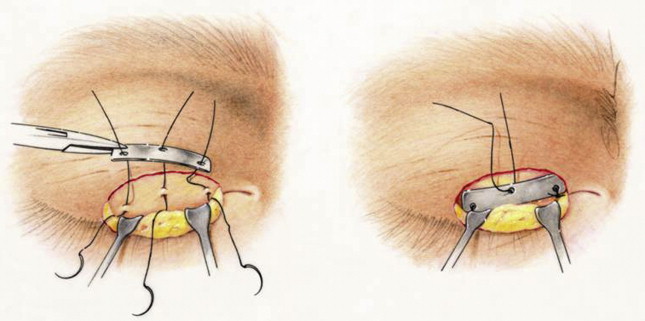
Lower lid malposition occurs less commonly in the pediatric population. In children with lower lid malposition, a tarsal strip may be performed ( Fig. 3 ). The patient is placed in a supine position, and the lower lid is injected with 1% lidocaine with 1:100,000 epinephrine. A lateral canthotomy and inferior cantholysis is performed. The gray line is denuded. The tarsal plate is deepithelialized, and a segment of the tarsal plate is trimmed. The tarsal plate is resuspended to the periosteum of the superolateral orbital rim. The incision is then closed. Other techniques described to elevate the lower eyelid include fascia lata slings, minitendon palmaris longus grafts, and conchal cartilage implants. As with platinum weights, older children and teenagers may tolerate tarsal strip as an office procedure, and younger children require general anesthesia.
Dynamic procedures
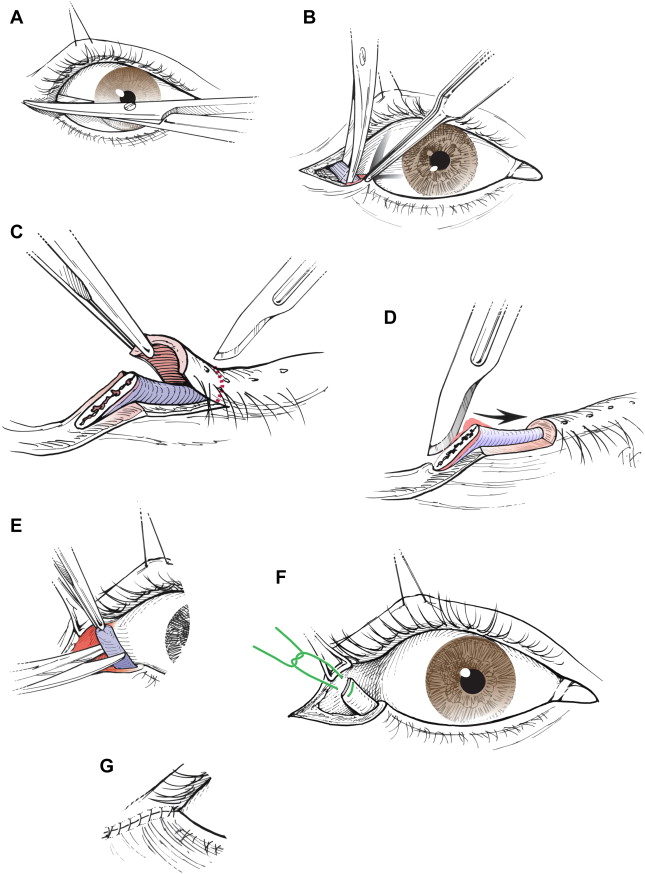
Dynamic procedures are required for restoration of the blink reflex. Terzis and Karypidis investigated dynamic procedures, including nerve transfers and eye sphincter substitution, in pediatric patients with facial paralysis. The nerve transfers included cross-face nerve grafting with microcoaptations to the affected facial nerve, minihypoglossal nerve transfers, and direct orbicularis oculi neurotization. Direct neurotization involved a cross-face nerve graft from selected branches of the unaffected facial nerve. The distal end of the interposition nerve graft was divided into several fascicles and implanted directly into the epimysium of the upper and lower orbicularis oculi muscle. Pedicled frontalis and temporalis muscles were used for regional eye sphincter substitution; free platysma and pectoralis minor muscles were used as free flaps for eye sphincter substitution. Overall, dynamic procedures significantly improved the blink scores, with direct orbicularis oculi muscle neurotization being significantly superior among the nerve transfers. In cases of inadequate but apparent facial movement or findings of partial innervation on needle electromyography (EMG), direct muscle neurotization is indicated. In complete paralysis, cross-face nerve graft with microcoaptations to the affected nerve may be performed. If muscled denervation time exceeds 27 months, muscle substitution for reestablishment of dynamic function has been suggested by some institutions, but is not common practice. Blink restoration is less frequently used than static techniques; however, dynamic procedures should be considered in pediatric patients with severe ocular involvement.
Nasal Rehabilitation
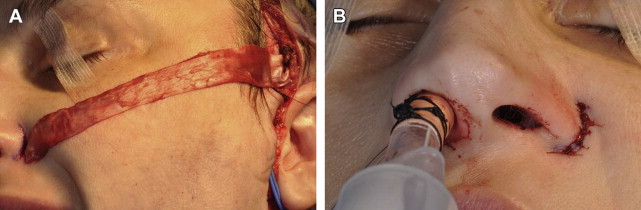
Children with facial paralysis may have external nasal valve collapse and nasolabial fold abnormalities. If the nasal base is severely deviated away from the paralyzed side, this is corrected with a static fascia lata sling. The patient is placed in a supine position. Fascia lata is harvested from the lateral thigh. A subcutaneous tunnel is created from a preauricular incision to an incision in the alar crease, and the fascia lata is secured medially. The appropriate position of the external nasal valve is adjusted, and the lateral portion of fascia lata is sutured to the temporalis fascia ( Fig. 4 ). Patients with continued nasal valve collapse are candidates for functional rhinoplasty. Effacement or hyperprominence of the nasolabial fold is addressed through static suture suspension techniques ( Fig. 5 ).
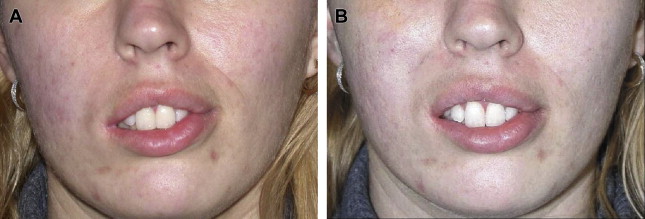
Smile Rehabilitation
One of the most critical components of pediatric facial reanimation is restoration of the smile. Although static facial suspension remains an option for children, dynamic reanimation is always preferred when possible. Dynamic rehabilitation can be accomplished with regional muscle flaps or free muscle transfers. Among regional flaps, the temporalis muscle is most frequently used to treat facial paralysis, because of its favorable vector pull and low morbidity. Leboulanger and colleagues published a small series of children with congenital facial palsy who were treated with temporalis transposition for smile rehabilitation. Eighty percent of children experienced adequate smile excursion, and the investigators concluded that the temporalis flap is an alternative treatment to free muscle transfer in children with congenital facial paralysis, citing the benefits of a single-stage operation, favorable scar, and immediate results. The disadvantages of temporalis flaps include lack of spontaneous smile, increased midfacial bulk, and a visible depression over the temporal fossa.
Although some investigators describe the use of regional flaps, free muscle transfer has become the first-line treatment for healthy pediatric patients who desire smile reanimation. There are multiple options for free muscle transfer, although the gracilis, pectoralis minor, and latissimus dorsi have been used specifically in the pediatric population with good success. The gracilis muscle remains most surgeons’ preference for adult and pediatric dynamic smile reanimation, because of its reliable vascular pedicle, low donor site morbidity, ability for simultaneous harvest, and favorable muscular microarchitecture.
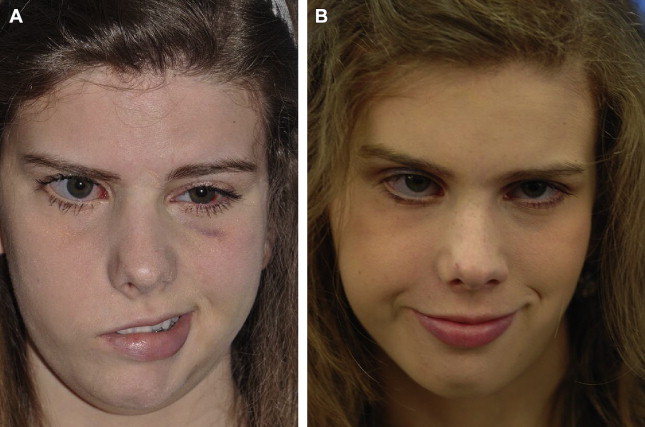
Two-stage procedures
Reanimation may be performed in 1 or 2 stages. The 2-stage procedure is preferred in children, unless the contralateral facial nerve is unavailable. In the 2-stage procedure, a cross-face nerve graft is coapted to selected branches of the facial nerve on the unaffected side and buried in the lip for 6 to 12 months. The sural nerve is the most frequently used nerve for the cross-face nerve graft. The gracilis muscle is then harvested, transferred to the paralyzed side, and driven by the cross-face nerve graft. The greatest benefit of the 2-stage procedure is the ability to restore spontaneous, emotive facial expression, which is the main goal of pediatric smile restoration ( Fig. 6 ). The major disadvantage is the higher failure rate compared with 1-stage procedures, resulting from inadequate penetration across the 2 neurorrhaphies, which occurs in approximately 20% of adults and children.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




