Chapter 46 Pathophysiology of the burn scar
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
Prehistoric and historic perspectives
Wounds due to combat, hunting injuries, accidents and thermal injuries have been the leading causes of death in humans for millennia. On the other hand, prolonged survival of large full-thickness wounds is a recent phenomenon. Several complex biological responses to injury to the skin have evolved over time, but there has been no evolutionary pressure to evolve appropriate responses to very large wounds. There are records of human attempts to improve wound healing in the most ancient texts from Mesopotamia and Egypt. Guido Majno has explored what can be learned from archeology and paleontology regarding wounds and their treatment in ancient times.1 He also provides a very accurate brief description of the process of wound healing in lay terms. In the development of modern medicine, the advances in wound treatment advocated by Ambroise Pare (1520–1590) stand out, together with the campaign for antisepsis of Joseph Lister (1827–1912), and the development of antibiotics as well as the development of the modern methods of treatment described in this book.
Incisional wounds with primary closure
The essential components of wound healing are easily understood. They are: (1) activation in the wound of tissue repair by phenotypic fibroblasts and small blood vessels, and an inflammatory response initiated by vascular leakage and (2) entry into the wound of circulating polymorphonuclear neutrophils, lymphocytes and macrophages.2 When a sharp incision is closed while still sterile, only minimal vascular leakage and inflammation occur, and the predominant reaction to injury occurs in the fibroblasts present in the dermis and subcutaneous tissue. These resting connective tissue cells are rapidly activated to secrete collagen, which quickly bridges the small remaining gap to restore the resistance of the skin to tearing. Vascular continuity is restored by budding and remodeling of blood vessels, and the basal keratinocytes of the epidermis divide briefly to restore the complete structure of the epidermal barrier. All that remains to indicate the site of injury is a linear ribbon of dense collagen with little flexibility that marks the site of the incision, as well as some small scars that mark the sites where sutures were inserted. This process of incisional wound healing was well described in humans by Russell Ross and his colleagues in Seattle.3,4
Delayed wound closure ‘by second intention’ and wound contraction
If the epidermis and dermis are incised or removed and the edges of the wound remain separated, much more prominent inflammatory and reparative events occur. Extensive leakage of blood plasma by damaged small blood vessels maintains an outward flow that serves to keep the wound clean, but causes deposition of coagulated fibrin and other desiccated proteins on the wound surface. Conversion of fibrinogen into fibrin fills the gap in the epidermis and provides a gelatinous matrix capable of sustaining migrating cells. Thrombin stimulates expression of interleukin-6 in connective tissue cells.5 Degranulation of platelets releases platelet-derived growth factor (PDGF) as well as several other proinflammatory cytokines.6 Degradation of fibrin releases peptides that stimulate fibroblast proliferation and secretion and division of vascular endothelial cells, and production of cytokines by other cells.7–9 The resting fibroblasts of the dermis, together with circulating stem cells, divide rapidly in the wound bed and secrete large quantities of collagen and proteoglycans, particularly those typically present in skin during fetal life, with predominance of type III collagen.10,11 Simultaneously, the endothelial cells of small blood vessels proliferate rapidly and form numerous small capillary loops that extend upwards toward the surface. Together, these cells form a mass of granulation tissue that covers the wound. All these activities of fibroblasts and endothelial cells are stimulated by cytokines and other peptides, largely secreted by monocytes and lymphoid cells that infiltrate the wound bed. Certain proteins and peptides that are normally present in blood plasma also stimulate and enable formation of the wound matrix, notably fibronectin and vitronectin.12–14 Polymorphonuclear neutrophils also enter the wound bed in large numbers, where they phagocytize and kill bacteria and fungi, which are always present in the outside world and gain entry to the dermis and subcutis through the open wound. Next, the fibroblasts develop interconnections among themselves and produce and assemble the contractile machinery of actin and myosin within the cytoplasm of each cell.15 These interconnected myofibroblasts contract to shrink the size of the open wound, pulling adjacent intact skin to cover the wound bed. This process of wound contraction is more dramatic in experimental wounds of rodents than in human wounds. The effects of wound contracture in applying tension to surrounding tissues, however, are sometimes very clear in humans as well. Simultaneously with these processes, the basal keratinocytes of the cut edges of the epidermis change to a migratory and secretory phenotype, and begin to invade the wound bed between the granulation tissue layer and the scab of dried proteins on the surface.16 Cell division of keratinocytes occurs near the cut edge of the wound to supply cells for this migration. Once they make contact to seal the center of the wound, the migrating keratinocytes change their phenotype again and restore the normal laminated structure of the epidermis and produce a new basal lamina.17,18 Melanocytes also develop migratory properties during healing of large wounds, since they establish a degree of pigmentation in the healed wound that approximates the pigmentation of the uninjured skin. It should be noted that only the epidermis regenerates to resemble the normal epidermis. Hair follicles, sweat glands and other epidermal appendages do not regenerate. Thus, the part of the wound that was not closed by contracture remains dry, hairless and flat. Furthermore, the restored dermis in a fully healed scar is composed of collagen type I fibers running in straight lines adjacent to one another parallel to the surface, providing good strength (though somewhat less than the native skin) but far less elasticity and flexibility than the connective tissue of the normal dermis.
First degree or superficial injury of skin
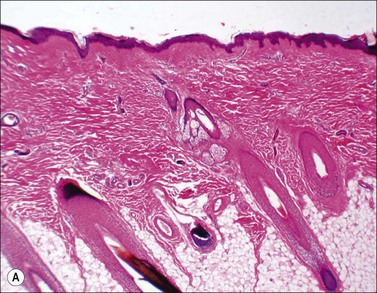
Superficial burn wounds are those in which part or all of the epidermis is lost, but the epidermal basal lamina remains intact and the dermis is uninjured. In these areas, only epidermal regeneration is required, hair follicles and sweat glands remain intact, and healing can occur with little or no disfigurement.19
Second degree or partial thickness injury
In partial thickness wounds, the entire epidermis and the upper part of the dermis become necrotic. If left intact, the presence of a large quantity of devitalized tissue requires prolonged activity of macrophages to clear the necrotic debris. Granulation tissue forms underneath the necrotic dermal tissue, and epidermal migration occurs under the eschar formed by dead tissue, leading to restoration of the epidermis, and production of dermal connective tissue in the form of a thin scar. The deep portions of the hair follicles remain viable, and the keratinocytes lining the hair follicles become migratory and undergo mitosis behind the migrating cells, eventually covering the surface with new epidermis derived from the hair follicle.16,20 In severe cases, loss of hair follicles may lead to insufficient regenerative activity to cover the surface.21–23 Recently, hair follicles have been found to contain several populations of multipotent stem cells that can generate cells that can multiply, migrate and regenerate the surface epidermis.24 The stem cell population that was first identified is slowly cycling, expresses the conventional stem cell surface marker CD34, and resides in the bulge region of the follicle near the attachment of the arrector pili muscle.25,26 More recently, additional stem cell populations have been identified that reside in the isthmus region and the hair germ region of the follicle and express distinct markers.27–29 Much is being learned about the function of these various groups of stem cells by studying knockout and overexpressing mice. The changes in follicular stem cells during healing of burn wounds, however, remain to be described.
One new aspect of hair follicle biology that is of great interest for burn surgeons is the delineation of the role of the dermal papilla, the tiny cluster of mesenchymal cells within the hair bulb.30 Fetal development of hair follicles apparently depends upon interaction between epithelial cells of the epidermis and mesenchymal cells. The mesenchymal cells of the dermal papilla, which can be amplified in culture using keratinocyte conditioned medium, can induce formation of hair follicles from interfollicular skin. Experiments have even been described, in which formation of new hair follicles, complete with hair, was induced in hairless nipple skin of the mouse, and in the renal capsule.31,32 Since one of the major problems in long-term care of burn patients is alopecia, the possibility that hair follicles could be caused to regenerate where there are none is very appealing.33
Third degree or full thickness injury
In full thickness burns, thermal injury extends deep enough to destroy all of the hair follicle that has the capacity to regenerate the epidermis, and some of the upper subcutaneous tissue may also become necrotic. In this case regeneration of the epidermis from hair follicles is not possible, and the wound can develop an epidermal covering only slowly, as the epidermis lateral to the wound spreads out over the entire wound surface.2 During this time, the necrotic tissue in the wound bed is at risk of infection, and extensive activity of tissue macrophages is required to eventually remove it.
Biology of wound healing
Granulation tissue and the proliferative phase of wound healing
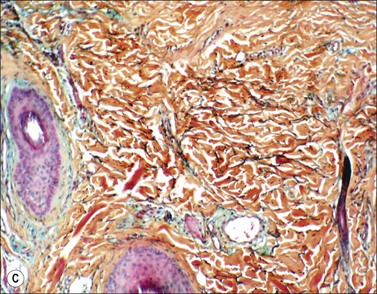
Massive proliferation of fibroblasts and vascular endothelial cells is characteristic of the early phase of wound healing. These cells, and the fine fibrils of collagen and the gel provided by multiple mucopolysaccharides and proteoglycans, make up the granulation tissue that is a major feature of all wounds that remain open. The most important of many peptides that stimulate fibroblast growth appear to be TGFβ and basic FGF, while the most important peptide that stimulates growth of endothelial cells appears to be VEGF.34–39 In order for wound contraction to occur, it is important for fibroblasts to form networks within the dermis that allow the wound to contract. As Donald Ingber has pointed out, interactions between the extracellular matrix and the cellular cytoskeleton are important in controlling cellular differentiation and function.40–42 Apparently, when wound healing is abnormal and hypertrophic scars develop, this process of interlinking of fibroblasts to form a meshwork that supports tension parallel to the surface is disrupted, since nodules of collagen result that do not flatten out normally.
Influx of circulating cells
Many circulating cells actively migrate into wound beds and play roles in defense against bacteria and fungi, clearance of devitalized tissue components, and stimulation of later phases of healing of the dermis and epidermis. Based on experiments with partially selective ablation of individual cell types, the cells most important in stimulating and maintaining tissue repair appear to be the T lymphocytes and monocytes.43 Monocytes, which differentiate into tissue macrophages, are responsible for synthesis and release of many of the cytokines important in wound healing, as are connective tissue cells and epithelial cells. In addition, recent research has clearly shown that circulating stem cells enter healing wounds, where they can differentiate to form fibroblasts and other connective tissue cells needed to restore tissue integrity.44,45
Migration of keratinocytes to cover the wound (epiboly)
When the epidermis is transected, changes take place rapidly in the basal cells of the epidermis adjacent to the wound. The process of epidermal regeneration has been well studied by Stenn and his colleagues.16,46,47 Altered basal keratinocytes send out thin sheets and undergo ameboid motion over the wound bed, but under the nonviable eschar and/or scab, secreting a provisional matrix as they go.48,49 Development of this migratory phenotype is stimulated by the plasma protein vitronectin, and requires the presence of albumin as a cofactor.16,47,50 Cell division occurs to support this migration, not among migratory cells, but among their precursors in the residual epidermis. Similar processes stimulate migration and replacement of epithelial cells from hair follicles in partial thickness wounds. After a sheet of epithelial cells is established over the entire wound surface, they begin to divide and eventually create a multilayered stratified squamous epithelium with a granular layer and keratinization. Epidermal cells secrete substantial quantities of interleukin-1β and other cytokines.51,52 The new proliferative epidermal basal cells also secrete a new basal lamina composed of laminin, type IV collagen and bullous pemphigoid antigen, adhere tightly to that basal lamina, and develop attachments of type VII collagen between the basal lamina and the underlying fibers of type I collagen in the scarred dermis. Also, just after the newly formed epithelial layer completely covers the wound, the phenotype of the connective tissue cells in the matrix undergoes a series of changes, and much less fibronectin is found in the wound matrix.17 As noted above, in second-degree burns, epidermal cells from the hair follicles regenerate the epidermis on the surface.
Collagen matrix formation and maturation
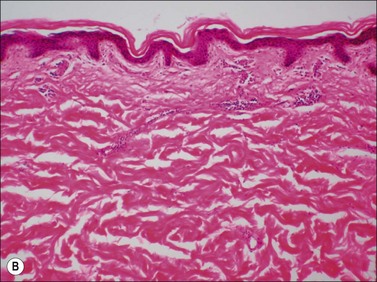
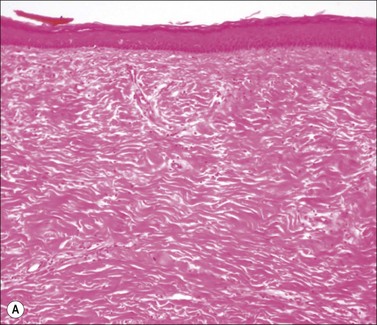
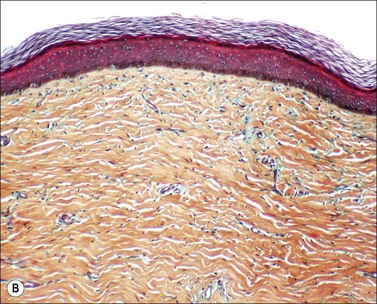
Scars gradually increase in their strength, as measured by their resistance to tearing, but never reach the strength of normal dermis. During this process, the delicate fibers of newly secreted collagens I and III are replaced by large collagen I fibers that are oriented parallel to each other and to the skin surface (Figs. 46.1, 46.2). Maturation of collagen fibers is largely a chemical process of remodeling that involves covalent crosslinking of adjacent polypeptide chains. Collagen fibers normally form and are degraded continuously in normal skin as well as in scars, but the processes that control the rates of formation and degradation and the orientation of the mature fibers are not fully understood.
Cytokines and growth factors
Many short polypeptides, mostly cytokines and growth factors, are responsible for the changes in cells that lead to wound healing and the termination of wound healing processes, and the formation and organization of a scar. Among the most important are the transforming growth factor beta family (TGFβ), basic fibroblast growth factor (bFGF or FGF2), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF).53–60 Frequently, the response of a particular cell type to a particular peptide depends not only upon binding of cell surface receptors by the right peptide, but also upon simultaneous signals from other cellular receptors. Thus the network of peptide signaling is complex, and the range of possible cellular responses is large enough to allow generation of complex structures and considerable fine-tuning.56,61 There are also many opportunities for reparative processes to go wrong, to proceed in an unbalanced fashion, or to fail to complete an appropriate cycle of activation and regression. This complex area has been well reviewed.62–64
Factors that alter wound healing
Changes in blood supply and perfusion
Development of methods to study local blood supply by ultrasonic measurement has led to the discovery that there is usually a zone of greatly increased blood flow below a burn wound, which is not surprising as part of a local inflammatory response to tissue injury. Above this zone of hyperemia is a zone of tissue ischemia, in which blood flow is less than normal. Remarkably, during the first 24 h after a burn wound, the zone of ischemia typically becomes significantly deeper, indicating that ischemic injury of dermal tissues actually leads to a depth of tissue necrosis greater than that produced by the immediate thermal injury.65 Experiments in animals have shown that neutrophils are involved in this progressive ischemic cell death in injured but viable tissue deep to a burn wound.66 Altered blood flow may lead to vascular thrombosis in a burn wound, also contributing to the risk of ischemic tissue injury. In normal skin, there is a plexus of arteries and veins immediately below the dermis in the upper layer of subcutaneous adipose tissue. This subdermal plexus is at risk of thrombosis in deep partial-thickness burns and in full-thickness burns, and it is vulnerable to damage in the process of tangential wound excision, with further loss of blood supply to the wound.