Introduction460
INTRODUCTION
Disease
Histopathological features
Erythema nodosum
Septal panniculitis with paraseptal inflammatory wedges; neutrophils early; septal giant cells; Miescher’s radial granulomas
Necrobiosis lipoidica
Fibrous septal widening; dermal granulomas and necrobiosis
Scleroderma
Fibrous septal widening; dermal or fascial thickening with parallel coarse collagen
Erythema induratum–nodular vasculitis
Lobular and septolobular panniculitis; vasculitis and granulomas; necrosis common
Subcutaneous fat necrosis of the newborn
Lobular panniculitis; needle-shaped clefts in fat cells
Sclerema neonatorum
Similar needle-shaped clefts (crystals), but no inflammation
Weber–Christian disease
Doubtful entity; lobular panniculitis; neutrophils early; abundant foam cells
α1-Antitrypsin deficiency
Lobular panniculitis; dermal liquefaction; suppuration; septal collagenolysis
Cytophagic histiocytic panniculitis
Lobular panniculitis; numerous histiocytes showing cytophagocytosis (‘bean bag’ cells)
Panniculitis-like T-cell lymphoma
Lymphocytic lobular panniculitis; pleomorphic lymphocytes of variable size which rim adipocytes; karyorrhexis; necrosis; sometimes angioinvasion and cytophagocytosis
Pancreatic panniculitis
Lobular panniculitis; ghost-like necrotic fat cells; peripheral nuclear dusting
Lupus panniculitis
Lobular panniculitis with prominent lymphocytes (lymphocytic lobular panniculitis); paraseptal lymphoid follicles in 50%; often basal lichenoid tissue reaction
Subcutaneous sarcoidosis
Lobular granulomas, ‘naked’ and tuberculoid; ± central necrosis; ± perigranulomatous fibrosis
Lipodystrophy
Subcutaneous atrophy; sometimes early mild lobular panniculitis
Lipedema
Hypertrophy of fat; thickened fibrous septa; edema and adipocyte degeneration
HIV-associated lipodystrophy
Non-inflammatory except for scattered lymphocytes and lipophages; atrophy of fat
Gynoid lipodystrophy
Lipohypertrophy with variable thickness of septa; fat herniated into lower dermis (may be gender-related)
Membranous lipodystrophy
Microcysts with lipomembranous (membranocystic) change; dense fibrosis between islands of ‘fatty microcysts’
Lipodermatosclerosis
Infarction of fat lobules; lipomembranous change; late fibrosis; hemosiderin; pericapillary fibrin caps; early inflammatory stage
Factitial panniculitis
Mixed septal and lobular panniculitis; sometimes suppurative; foreign body giant cells
Traumatic fat necrosis
Overlap with above; fat cysts, fibrosis and sometimes hemosiderin
Encapsulated fat necrosis
Lobules of necrotic fat; thin fibrous capsule which is usually hyaline; lipomembranous change common
Infective panniculitis
Suppuration or granulomas or numerous eosinophils depending on etiology
Non-infective neutrophilic panniculitis
Neutrophilic lobular panniculitis; necrosis sometimes present, admixture of cells with chronicity
Eosinophilic panniculitis
Numerous eosinophils in a lobular panniculitis; sometimes flame figures or parasite present
Calciphylaxis
Lobular fat necrosis with calcification; calcification in small vessels; mixed inflammatory infiltrate
Miscellaneous
Urate crystals in gout; necrobiotic granulomas in subcutaneous granuloma annulare or rheumatoid nodules
Panniculitis secondary to large vessel vasculitis
Vasculitis of artery or vein; panniculitis localized around vessel
SEPTAL PANNICULITIS
Erythema nodosum
Erythema nodosum migrans
Necrobiosis lipoidica
Factitial panniculitis (some)
Nephrogenic systemic fibrosis
Cellulitis
Microscopic polyangiitis
Hydroa vacciniforme (lobular also)
Apomorphine infusion
Cryoglobulinemia
Whipple’s disease
Cytomegalovirus infection
α1-Antitrypsin deficiency (rare cases)
ERYTHEMA NODOSUM
Histopathology
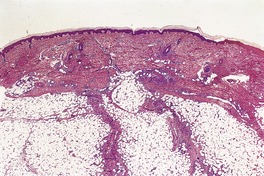
Fig. 17.1
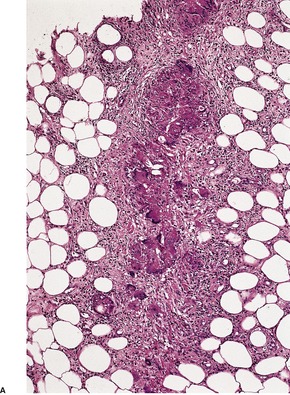
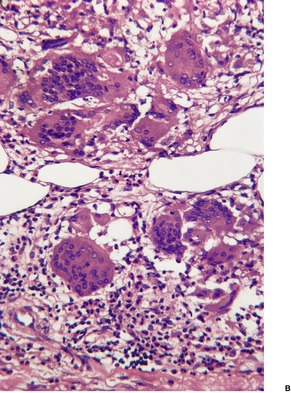
Fig. 17.2
SCLERODERMA
LOBULAR PANNICULITIS
ERYTHEMA INDURATUM–NODULAR VASCULITIS
Histopathology90.97.106.107. and 108.
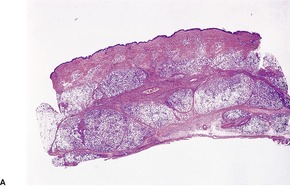
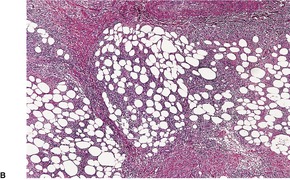
Fig. 17.3
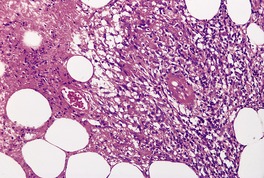
Fig. 17.4
SUBCUTANEOUS FAT NECROSIS OF THE NEWBORN
Histopathology112.129. and 130.
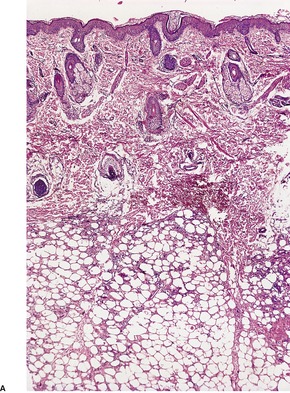
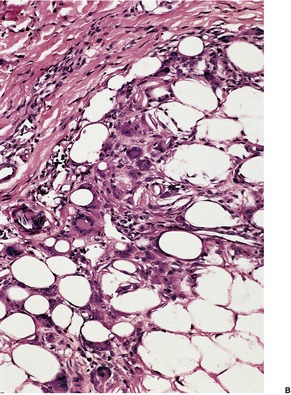
Fig. 17.5
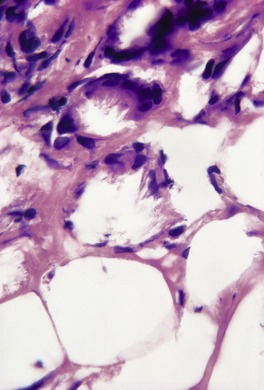
Fig. 17.6
SCLEREMA NEONATORUM
Histopathology
COLD PANNICULITIS
Histopathology
WEBER–CHRISTIAN DISEASE
Histopathology
α1-ANTITRYPSIN DEFICIENCY
Histopathology
CYTOPHAGIC HISTIOCYTIC PANNICULITIS
Histopathology
PANNICULITIS-LIKE T-CELL LYMPHOMA
Histopathology
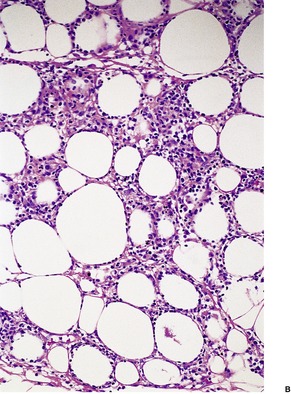
Fig. 17.7
PANCREATIC PANNICULITIS
Histopathology
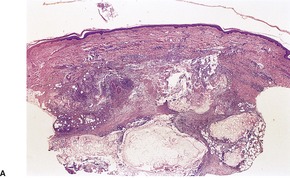
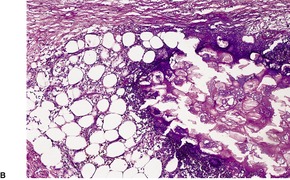
Fig. 17.8
LUPUS PANNICULITIS
Histopathology

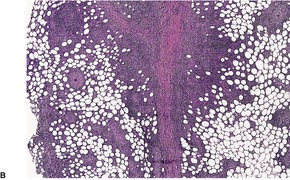
Fig. 17.9
CONNECTIVE TISSUE PANNICULITIS
Histopathology
POSTSTEROID PANNICULITIS
Histopathology
SUBCUTANEOUS SARCOIDOSIS
Histopathology
LIPODYSTROPHY SYNDROMES
Total lipodystrophy
Partial lipodystrophy
Localized lipodystrophy
Lipedema
Histopathology
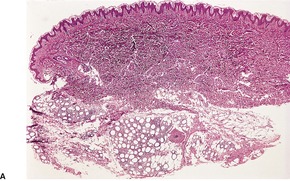
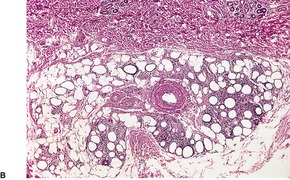
Fig. 17.10
HIV-ASSOCIATED LIPODYSTROPHY
Histopathology356
GYNOID LIPODYSTROPHY (CELLULITE)
Histopathology363. and 364.
MEMBRANOUS LIPODYSTROPHY
Histopathology242.370.371. and 372.
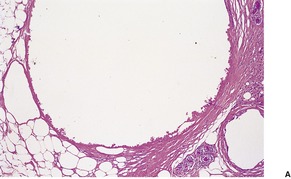
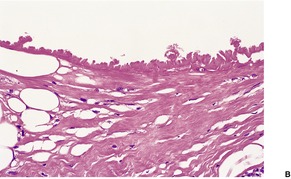
Fig. 17.11
LIPODERMATOSCLEROSIS
Histopathology
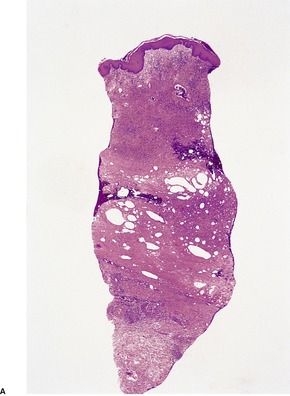
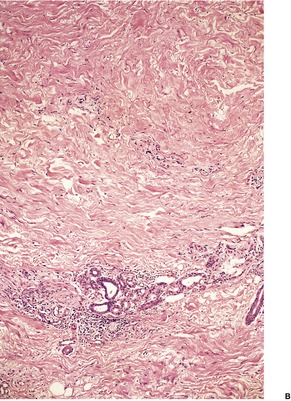
Fig. 17.12
FACTITIAL PANNICULITIS
Histopathology

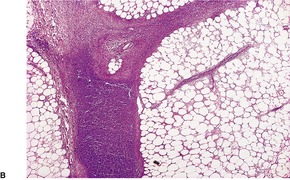
Fig. 17.13
POSTIRRADIATION PSEUDOSCLERODERMATOUS PANNICULITIS
Histopathology
TRAUMATIC FAT NECROSIS
Histopathology
ENCAPSULATED FAT NECROSIS
Histopathology
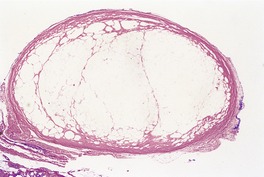
Fig. 17.14
INFECTIVE PANNICULITIS
Histopathology423
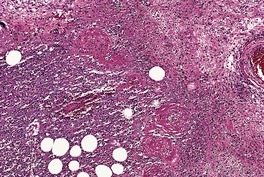
Fig. 17.15
NEUTROPHILIC PANNICULITIS
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Panniculitis
Lobular panniculitis463
Erythema induratum–nodular vasculitis463
Subcutaneous fat necrosis of the newborn463
Sclerema neonatorum464
Cold panniculitis464
Weber–Christian disease465
α1-Antitrypsin deficiency465
Cytophagic histiocytic panniculitis466
Panniculitis-like T-cell lymphoma466
Pancreatic panniculitis467
Lupus panniculitis468
Connective tissue panniculitis468
Poststeroid panniculitis469
Subcutaneous sarcoidosis469
HIV-associated lipodystrophy471
Gynoid lipodystrophy (cellulite)471
Membranous lipodystrophy471
Lipodermatosclerosis472
Factitial panniculitis473
Postirradiation pseudosclerodermatous panniculitis474
Traumatic fat necrosis474
Encapsulated fat necrosis474
Infective panniculitis474
Neutrophilic panniculitis475
Eosinophilic panniculitis475
Calciphylaxis476
Miscellaneous lesions476
The panniculus adiposus (subcutaneous fat) is a metabolic depot which also functions as a layer of insulation and as a buffer to trauma. It is composed of mature lipocytes, which are round to polygonal cells with an eccentric nucleus and a large cytoplasmic lipid vacuole. 1 In contrast, fetal fat cells contain multiple small lipid vacuoles. The lipocytes are separated from their neighbors by an inconspicuous matrix. 2
The panniculus adiposus is divided into lobules by fibrous septa, which are continuous with the dermis. Smaller microlobules have been described within the larger lobules, but these smaller units are not of pathological importance. Within the fibrous septa run the small arteries and arterioles, venules, lymphatics, and nerves. The nutrient artery supplies the center of the lobule with drainage to venules in the fibrous septa. 2 As a consequence, interference with the arterial supply results in diffuse changes within the lobule (lobular panniculitis) while venous disorders are manifested by alterations in paraseptal regions (septal panniculitis). 2
This chapter is concerned primarily with the inflammatory lesions of the subcutaneous fat – the panniculitides. Miscellaneous infiltrates and deposits will be mentioned briefly.
Inflammatory lesions of the subcutaneous fat can be classified into three distinct categories:
• septal panniculitis
• lobular panniculitis
• panniculitis associated with large vessel vasculitis.
Within each group, the histological appearances will depend on the stage of the disease at which the biopsy is taken. In early lesions there are often neutrophils in the inflammatory infiltrate, while later lesions have a chronic infiltrate composed predominantly of lymphocytes but with a variable admixture of giant cells and lipid-containing macrophages. At an even later stage there is some fibrosis. Accordingly, attempts at subclassifying the panniculitides on the basis of the predominant pattern of inflammation or cell type are largely unsuccessful, except for those with abundant eosinophils.
Inflammation of small venules will result in a septal panniculitis with some spillover of the inflammatory process into the lower dermis. 2 Involvement of the arterial supply, for example by vasculitis, will produce a lobular panniculitis. There are other mechanisms involved in some of the diseases that result in a lobular panniculitis. These will be considered in the appropriate sections. The panniculitis associated with large vessel involvement, such as polyarteritis nodosa and migratory thrombophlebitis, is usually localized to the immediate vicinity of the involved vessel. It often has mixed lobular and septal features.
Unless an adequate biopsy is received, it may be difficult to reach a specific diagnosis.3. and 4. A diagnosis of ‘lobular panniculitis ? type’ may have to suffice in these cases. Another problem with the panniculitides is the considerable confusion that exists in the literature. 5 In some reports it would seem that a diagnosis has been made on purely clinical grounds and the pathological findings ignored. Several reviews of the panniculitides have recently been published. They describe the diversity of histological appearances that can be seen in the various panniculitides.6.7.8. and 9.
The histological features of the panniculitides are summarized in Table 17.1.
In septal panniculitis the inflammatory reaction is centered on the connective tissue septa of the subcutaneous fat. 7 If the lesions are of long standing or recurrent in the same area, the septa may be considerably widened with a corresponding reduction in the amount of intervening lobular fat. There is often some spillover of inflammatory cells and macrophages into the adjacent fat lobule. In small biopsies this spillover of inflammatory cells can be misinterpreted as a lobular panniculitis.
In addition to the diseases considered below, a septal panniculitis may be seen in some cases of factitial panniculitis (see p. 473), cellulitis (see p. 552), nephrogenic systemic fibrosis (see p. 358), 10 microscopic polyangiitis (see p. 216), and hydroa vacciniforme (see p. 536). The septal panniculitis reported in a patient with cytomegalovirus infection was probably an infective panniculitis and not erythema nodosum. 11 It has also been recorded in a patient with cryoglobulinemia (see p. 200) and in patients receiving apomorphine infusion for Parkinson’s disease. 12 The symmetrical panniculitis reported in a patient with Whipple’s disease showed a septal panniculitis with numerous PAS-positive foamy macrophages infiltrating the septa. 13
The causes of septal panniculitis are listed in Table 17.2.
Erythema nodosum is an acute, painful, erythematous, nodular eruption. The nodules, which range from 1 to 5 cm or more in diameter, are usually situated on the anterior aspect of the lower legs; more rarely, they occur on the arms, soles,14.15. and 16. or trunk. There may be associated fever, malaise, and arthralgia. The lesions subside after a period of approximately 2–6 weeks or so. Up to one-third of cases may recur. Erythema nodosum usually occurs in young adults, but childhood cases occur. 17 Encapsulated fat necrosis (‘mobile encapsulated lipoma’) is a rare complication (see p. 474). 18 Erythema nodosum may be associated with sarcoidosis, 4 Sweet’s syndrome,19. and 20. Behçet’s disease,21.22. and 23. celiac disease, 24 inflammatory bowel disease, intestinal bypass syndrome, 25 eosinophilic esophagitis, 26 carcinomas,27. and 28. carcinoid tumor, 29 leukemia, 30 lymphoma,31.32. and 33. hairy cell leukemia, 34 tularemia35 or with streptococcal, 4 staphylococcal, 36 meningococcal, 37 campylobacter, 38 giardia, 39 salmonella, 40 histoplasma, 4 mycoplasma,41. and 42. chlamydial, 43 mycobacterial,44. and 45. dermatophyte, 46 sporotrichosis, 47 human immunodeficiency virus, 48 or yersinia49. and 50. infections. Various drugs have been incriminated, 51 including isotretinoin, 52 sulfonamides, penicillin, ciprofloxacin, 53 minocycline, 54 leukotriene-modifying drugs such as zileuton, 55 azathioprine, 56 salicylates, iodides, gold salts, echinacea, 57 hepatitis B vaccine, 41 capecitabine, 58 and also oral contraceptives. 59 The triad of erythema nodosum, bilateral hilar lymphadenopathy, and polyarthralgia (Lofgren syndrome) represents an acute, benign, and usually self-limiting form of sarcoidosis. 60 It is said to be the most common form of sarcoidosis in Spain. The erythema nodosum is present at the onset in most cases. 60 Radiotherapy may also trigger the onset of erythema nodosum.61. and 62. In about one-third of cases the etiology is obscure.
The pathogenesis of erythema nodosum is unknown but it may represent an allergic response to infection or systemic disease. Reactive oxygen intermediates, released by primed neutrophils, may play a role in the pathogenesis. 63
In erythema nodosum migrans (subacute nodular migratory panniculitis), a centrifugally enlarging plaque with central clearing develops on the lower leg. It is usually solitary, but several lesions may exist.64.65.66.67.68. and 69.
In chronic erythema nodosum there are non-ulcerated lesions located mainly on the legs which may persist for years. There is no migratory tendency. 70
Treatment for erythema nodosum includes non-steroidal anti-inflammatory drugs, corticosteroids, and potassium iodide. Chronic cases have been successfully treated with infliximab71 and with adalimumab. 70
Erythema nodosum is a septal panniculitis with small foci of inflammatory cells extending into the adjacent lobular fat (Fig. 17.1). In some cases this spillover of cells is more marked and includes foam cells, sometimes associated with focal necrosis of fat cells adjacent to the septa. Lobular extension can be quite marked in the fasciitis–panniculitis associated with brucellosis. 72 The center of the lobule is spared, allowing a distinction to be made from lobular panniculitis. There is also some extension of inflammatory cells into the adjacent lower dermis. In most biopsies the septal infiltrate is predominantly lymphocytic, but there are variable numbers of giant cells, usually of foreign body type, as well as a few eosinophils and histiocytes (Fig. 17.2). Small nodules composed of spindle to oval histiocytes arranged around a minute slit may be found (Miescher’s radial granulomas).7. and 73. Well-formed tuberculoid granulomas are rare.
Erythema nodosum. The inflammatory infiltrate is confined to the septa of the subcutis. (H & E)
(A) Erythema nodosum. (B) The inflamed septum contains a number of multinucleate giant cells in addition to lymphocytes and eosinophils. (H & E)
In early lesions neutrophils are usually present. They are numerous in the rare suppurative variant of erythema nodosum in which neutrophils extend into the adjacent lobule.74. and 75. The fibrous septa are widened with edema and some fibrinoid change in the earlier stages. Later there are increased numbers of fibroblasts with some fibrosis.
Blood vessel changes are variable. There is usually prominent endothelial swelling of small septal vessels and sometimes of medium-sized veins. There is lymphocytic cuffing of septal venules. Sometimes a definite vasculitis is present, but by the time most biopsies are taken, only non-specific vascular changes are found. In the erythema nodosum-like lesions of Behçet’s disease, a vasculitis is present in most cases. It may be of lymphocytic or leukocytoclastic type. 76 The lesions are not always confined to the septa. 76
Thurber and Kohler have stressed the variability in the histological appearances of erythema nodosum. 77 They presented four cases that initially showed unusual features, but which, on subsequent biopsies showed typical findings. Two cases initially showed predominantly neutrophilic infiltrates with focal suppuration, as well as vasculitis of medium sized arteries. The third case showed a lobular panniculitis with a predominantly lymphohistiocytic infiltrate, while the fourth case showed a mixed septal and lobular panniculitis with a polyclonal lymphohistiocytic infiltrate and a vasculitis. 77
In a case reported as ‘neoplastic erythema nodosum’, a B-cell lymphoma produced both a lobular and a septal panniculitis. 32 It is not erythema nodosum.
In erythema nodosum migrans (subacute nodular migratory panniculitis) the septa are markedly thickened and fibrotic. Inflammation is usually mild although multinucleated giant cells and granulomas may be conspicuous along the edge of the septa. Neovascularization is often present at the septal borders.
In scleroderma and its localized cutaneous variants (morphea) there may be extension of the process from the dermis into the subcutaneous fat. There is marked fibrous thickening of the septa, and often lymphoid collections are present at the junction of the thickened septa and the fat lobules. 79
There are variants of morphea in which only the subcutaneous fat and/or fascia are involved. The terms subcutaneous morphea and morphea profunda are often used interchangeably for these variants, although morphea profunda has been proposed as the appropriate diagnosis when both fat and fascia are involved and subcutaneous morphea for cases with involvement of fat only (see p. 306). A panniculitis usually accompanies the fasciitis associated with eosinophilic fasciitis. 80 Septal fibrosis and hyalinization are obvious features. There are often small lymphoid collections but not lymphoid follicles with germinal centers. Plasma cells are usually present. They were the predominant cell type in two siblings with the disease. 81
A case of linear scleroderma with an intense plasma cell infiltrate in the dermis and subcutaneous fat has been reported. 82 Nodular aggregates of plasma cells were present in the fat lobules and the thickened interlobular septa. Scleroderma is considered further in Chapter 11 (pp. 304–310).
In the lobular panniculitides, the inflammatory infiltrate is present throughout the lobule but there is often some septal involvement as well. There are many different causes of a lobular panniculitis and many of these are histologically distinct. 8 However, sometimes it is not possible to distinguish between the various etiological groups, in which case the histological diagnosis may have to be simply ‘lobular panniculitis’.
Erythema induratum and nodular vasculitis were originally regarded as one entity. However, it has been the practice for the last 50 years or so to recognize two variants – one of presumptive tuberculous origin (erythema induratum, Bazin-type) and the other of non-tuberculous origin, known as nodular vasculitis or erythema induratum, Whitfield-type. 3 The tuberculous group was regarded as a tuberculid (see p. 559) on the basis of the persistent failure to culture Mycobacterium tuberculosis from lesional tissue or to see organisms on acid-fast stains. The tuberculous origin of the Bazin subtype was assumed on the basis of strongly positive reactions with the Mantoux test, the presence of active tuberculosis in other organs in some cases, and the favorable response of the skin lesions to antituberculous therapy.83.84.85.86.87. and 88.
The advent of PCR-based methods has led to the detection of mycobacterial DNA in from 30 to 80% of cases of erythema induratum–nodular vasculitis, defined on the basis of clinical presentation and a lobular granulomatous panniculitis on histology.89.90.91.92.93. and 94. However, a study of 10 cases in 2005, failed to demonstrate Mycobacterium of any species by PCR techniques. 95 As no significant differences have been consistently demonstrated between cases with detectable mycobacterial DNA and those without, it does not seem tenable to continue the separation of erythema induratum and nodular vasculitis. 96 After all, erythema nodosum has multiple etiologies (including tuberculosis), and it is considered as one condition.44. and 97. Thus the wheel has turned full circle and once again there is a single (composite) entity. It has been suggested that patients presenting with this disease should have an ELIspot (T-SPOT.TB) assay for detecting active or latent tuberculous disease. 98
Erythema induratum–nodular vasculitis is characterized by recurrent crops of tender erythematous nodules, usually with a predilection for the calves, although the shins are sometimes involved as well. The nodules may coalesce to form one or more plaques. Uncommonly, lesions may develop in other sites such as the buttocks and arms. Although it was originally regarded as a disease of young adult females, a wide range of ages can be affected. Lesions occasionally ulcerate; they may heal with scarring. 99
Erythema induratum–nodular vasculitis appears to be a disease of diverse etiologies, mycobacterial infection being one of them. Presumably other infections may be involved. To date, only hepatitis C has been incriminated.100.101. and 102. An association with ulcerative colitis has been reported. 103 Both erythema nodosum and a lobular panniculitis with vasculitis can be produced by brucellosis. 104 A paraneoplastic presentation has also been suspected. 105 The pathogenesis appears to involve a delayed hypersensitivity or Arthus-type reaction. Host responses may perpetuate the disease even after the infection has been cleared. 90
In cases suspected of having a tuberculous etiology, antituberculous therapy should be given. 88
The inflammatory changes are usually restricted to the subcutis and the lower dermis. There is a lobular or septolobular panniculitis, usually diffuse, with varying combinations of granulomatous inflammation, vasculitis, focal necrosis, and septal fibrosis (Fig. 17.3). As in other panniculitides the histological changes will vary with the duration of the lesions.
(A) Erythema induratum–nodular vasculitis. (B) There is inflammation in the fat lobules and to a lesser extent in the septa. (H & E)
Granulomas may be well developed and tuberculoid; occasionally they show caseation necrosis. More often the granulomas are poorly developed. Lipophage collections are usually present. The inflammatory infiltrate includes neutrophils, lymphocytes, and some plasma cells. Neutrophils predominate in areas of fat necrosis.
Vascular changes, which are present in approximately 90% of cases, involve all sizes of arteries and veins. 109 Involved vessels show endothelial swelling and a mixed inflammatory cell infiltrate in the wall and periadventitial tissues (Fig. 17.4). A necrotizing vasculitis is sometimes present, particularly in early lesions.
Erythema induratum–nodular vasculitis. There is a lobular panniculitis with focal necrosis. A small blood vessel shows fibrinoid change. (H & E)
Although no histological feature consistently reflects a tuberculoid etiology, necrosis is slightly more common in this group. In the past, the presence of tuberculoid granulomas in the deep dermis, often around the eccrine glands, was regarded as suggestive of a tuberculous etiology. 110 However, it is the absence of consistent differences that has resulted in the concept of a unified entity. 110
As a rule, erythema induratum–nodular vasculitis involves many contiguous lobules, whereas cutaneous polyarteritis nodosa produces a more localized panniculitis restricted to the vicinity of the involved vessels.
Subcutaneous fat necrosis of the newborn is a self-limited condition, present at birth or appearing in the first few days of life.111.112. and 113. It is characterized by indurated areas and distinct nodules with a predilection for the cheeks, shoulders, buttocks, thighs, and calves. 114 Sometimes only solitary lesions are discernible. Lesions may be painful. Subcutaneous atrophy is a common complication. 115 Hypercalcemia has been reported in some cases.116.117.118.119. and 120. Thrombocytopenia, hypoglycemia, and hypertriglyceridemia may also develop. 121 Lactic acidosis, and hyperferritinemia were each present in one case.122. and 123. Obstetrical trauma, hypothermia, asphyxia, and anemia have been incriminated in the etiology. 124 A recent study from Paris added failure to thrive, forceps delivery, macrosomia, and exposure to active or passive smoking during pregnancy. 115 Cases have been reported after hypothermic cardiac surgery,125. and 126. prostaglandin E administration, and maternal exposure to cocaine or calcium channel blockers.127. and 128. It has been suggested that trauma to fragile adipose tissue low in oleic acid and with a compromised circulation, followed by the release of hydrolases, leads to the breakdown of unsaturated fatty acids. 111 It should be remembered that infant fat already has a greater ratio of saturated to unsaturated fatty acids than exists in adult fat.
There is a normal epidermis and dermis with an underlying lobular panniculitis. Focal fat necrosis is present and this may lead to fat cyst formation. There is an inflammatory infiltrate of lymphocytes, histiocytes, foreign body giant cells, and sometimes a few eosinophils wedged between the fat cells (Fig. 17.5). Many of the fat cells retain their outline but contain fine, eosinophilic cytoplasmic strands and granules, between which are narrow clefts radiating from a point near the periphery of the cell (Fig. 17.6). 131 The clefts contain doubly refractile crystals, representing triglycerides, on frozen section. Similar fine, needle-like crystals can be seen in relation to some of the giant cells. Cases have been reported without the needle-like crystals. 132 Eosinophilic granules, presumably released from surrounding degranulating eosinophils, have been described in the cytoplasm of the multinucleated giant cells.131. and 133. In older lesions there is some fibrosis between the fat cells and there may be foci of calcification.
(A) Subcutaneous fat necrosis of the newborn. (B) There is a lobular and paraseptal panniculitis with lymphocytes, macrophages, and multinucleate giant cells wedged between the fat cells. (H & E)
Subcutaneous fat necrosis of the newborn. Narrow strands of tissue radiate from a point near the periphery of several of the fat cells. The intervening clefts represent the site of deposition of ‘fat crystals’. (H & E)
Sclerema neonatorum has a pathogenetic relationship to subcutaneous fat necrosis of the newborn and was at one stage regarded as a diffuse form of the latter. 134 It is also characterized by intracellular microcrystallization of triglyceride in the subcutaneous and sometimes also in the visceral fat of preterminal neonates. Sclerema neonatorum produces wax-like, hard skin which is also dry and cold. It is rarely seen these days, presumably because of improved neonatal care.
There are fine, needle-like crystals in the fat cells, but unlike subcutaneous fat necrosis of the newborn, there is very little inflammation, few giant cells, and no calcification. The subcutaneous septa are often widened by edema which might explain the ‘wide intersecting fibrous bands’ formerly reported. Fat cells have been reported as increased in size, but a personally studied case showed small, immature fat cells.
Panniculitis may occur following exposure to severe cold, particularly in infants.135.136.137. and 138. It has occurred in older children and on the thighs of women who have ridden horses in cold weather.139. and 140. In the latter instance it has been suggested that tight pants may restrict the blood supply, contributing to the injury. The lesions are indurated, somewhat tender plaques and nodules.
There is a lobular panniculitis with a mixed inflammatory cell infiltrate. Changes are most marked near the dermosubcutaneous junction where the vessels show a perivascular infiltrate of lymphocytes and histiocytes. There is some thickening of vessel walls. There is overlap with the changes described in deep perniosis (see p. 235). 141
The diagnosis of Weber–Christian disease has engendered more confusion in dermatopathology than practically any other.142.143.144.145.146.147.148.149.150.151.152.153.154.155.156. and 157. It has been applied by some to all cases of panniculitis with systemic symptoms or to all cases of fatal panniculitis with visceral involvement. 143 The term ‘Weber–Christian disease’ has been a convenient ‘pigeon hole’ for undiagnosable cases of panniculitis. 158
Weber–Christian disease was defined in the past as a rare disorder with recurrent subcutaneous nodules and plaques with a predilection for the extremities and buttocks, commonly associated with fever. 154 It was said to have a short self-limited course or follow an unremitting course with a fatal outcome and visceral involvement.151.155. and 156.
In a review of 30 cases diagnosed as Weber–Christian disease at the Mayo Clinic, White and Winkelmann were able to reclassify the cases as other entities, such as erythema nodosum, factitial panniculitis, cytophagic histiocytic panniculitis, and post-phlebitic syndrome. 159The diagnosis should no longer be used.
The term Rothman–Makai syndrome was regarded as a rare variant of Weber–Christian disease, but without the fever and systemic symptoms. Despite a recent case report, this entity is thought to no longer exist. 160
Weber–Christian disease was diagnosed in the past as a lobular panniculitis with numerous lipophages. It is this latter feature, now known to occur in other panniculitides, that often took precedence as a marker of the condition.
α1-Antitrypsin deficiency (OMIM 107400) is a genetic disorder characterized by low serum levels of α1-antitrypsin. The gene (PI) encoding this protease inhibitor is situated at 14q32.1. There are at least 90 allelic variants of this condition. Twenty-eight of the 40 cases (70%) of panniculitis in which the phenotype had been determined (up to 2004) had the ZZ phenotype, but other phenotypes have been involved.157.161.162. and 163. Panniculitis may be an early sign of this deficiency, but most cases present during the third and fourth decades. 164 The lesions begin as tender, erythematous, indurated, subcutaneous nodules that may be widely disseminated on the trunk, particularly the buttocks, or on extremities, especially the proximal limbs.147.149.162. and 164. They may be precipitated by childbirth, and by trauma, including cryotherapy.165. and 166. It has been associated with the extravasation of clarithromycin. 167 Spontaneous ulceration with discharge of an oily fluid may occur. Ulceration is uncommon in the other panniculitides.
Different treatments have been used to control the cutaneous lesions, such as corticosteroids, dapsone, cyclophosphamide, colchicine, and doxycycline. 168 Two more recent therapies have included plasma exchange or a purified concentrate of α1-antitrypsin (Prolastin®).168.169. and 170.
There is usually an acute panniculitis, sometimes septal as well as lobular, with masses of neutrophils and some necrosis of fat cells.171.172. and 173. Neutrophils usually extend into the reticular dermis, producing a characteristic infiltrate between the collagen bundles (so-called ‘splaying of neutrophils’). 174 Dissolution of dermal collagen with transepidermal elimination of ‘liquefied’ dermis may occur. There may also be collagenolysis of the fibrous septa of the subcutis, resulting in isolated adipocyte lobules. 149 Occasionally lobular panniculitis with fat necrosis alone occurs. Rarely, a septal panniculitis with a mixed infiltrate with a predominance of neutrophils occurs. 162 Destruction of elastic tissue may also be present. 147 Vasculitis is sometimes present in the subcutis. Later lesions show collections of histiocytic cells and lipophages and variable fibrosis. Dystrophic calcification may develop. 157
Winkelmann and colleagues coined the term ‘cytophagic histiocytic panniculitis’ over 25 years ago for a usually fatal syndrome which included a chronic and recurring panniculitis with an infiltrate of cytophagic histiocytes with eventual multisystem involvement, terminating usually with a hemorrhagic diathesis resulting from the hemophagocytic syndrome.145.175.176.177.178.179.180.181.182.183.184.185. and 186. Other cases have been included in the past with systemic Weber–Christian disease. 144 The current status of this condition is controversial. Two distinct entities appear to be included under this ‘umbrella’ term: subcutaneous panniculitis-like T-cell lymphoma (see below); and an authentic panniculitis for which the term cytophagic histiocytic panniculitis still applies. In the latter group are several patients in whom the disease has not progressed to overt lymphoma after prolonged follow-up (41 years in one case).187. and 188. Furthermore, monoclonal T-cell populations cannot be detected in these cases, although parallels have been drawn with large plaque parapsoriasis in which this may also occur. 189 It is possible that isolated cases represent a manifestation of a non-neoplastic, infection-associated hemophagocytic syndrome.189.190. and 191. Fatal cases still occur in the presumptive non-lymphoma group. 192
The disease, as originally described, consists of ulcerating nodules or plaques, sometimes painful, with a predilection for the legs and forearms. Other sites are often involved in established cases. It affects mostly middle-aged or elderly individuals.
There is a lobular panniculitis, sometimes with extension into the lower dermis. 145 There may be fat necrosis, focal hemorrhage, and a non-specific inflammatory infiltrate. The diagnostic feature is the presence of sheets and clusters of histiocytes showing prominent phagocytosis of red cells, white cells, and nuclear debris. Cells stuffed with phagocytosed materials have been called ‘bean bag’ cells. 145 Membranocystic change is uncommonly present. 193
Panniculitis-like T-cell lymphoma is a rare form of cutaneous lymphoma; over 150 cases have now been reported.194.195.196.197. and 198. Affected individuals present with multiple subcutaneous tumors or plaques, often associated with constitutional symptoms. Unusual presentations include patches of alopecia199 and venous stasis-like ulceration. 200 Most cases occur in adults but children can be affected.201. and 202. Sometimes a cytokine-induced hemophagocytic syndrome develops.188. and 194. The disease is indolent in some,195. and 199. but it may run a rapid course. Most cases have the phenotype of cytotoxic T cells and are derived from αβ T cells. 203 A case purporting to have resulted from previous chemotherapy has been reported. 204 Another case developed in a cardiac allograft recipient. 205
A panniculitis has also been reported in association with a lymphoma composed of natural killer cells. 206 An association with Epstein–Barr virus is common in these CD56+, nasal-type lymphomas.194.207. and 208. They have an aggressive course, often terminating with a fatal hemophagocytic syndrome. 209 This variant is derived from γδ T cells. 210 They bear a plasmacytoid dendritic cell phenotype. 211
These types of cutaneous lymphoma are considered further in Chapter 41 (p. 984).
There is a variable admixture of pleomorphic small, medium or large lymphocytes and histiocytes infiltrating the subcutis in the pattern of a lobular panniculitis (Fig. 17.7). 212 Neoplastic cells rim individual adipocytes in a lace-like pattern. 203 This feature is not specific for this entity but can also be seen in other lymphomas with cutaneous involvement. 213 Fat necrosis and karyorrhexis occur in all cases, while cytophagocytosis and angioinvasion are sometimes seen. 214
(A) Panniculitis-like T-cell lymphoma. (B) There is a lobular panniculitis. There is atypia of lymphocytes but this diagnosis is often missed in early lesions. (H & E)
In the nasal-type lymphoma of CD56+ natural killer cells, angioinvasion and necrosis are common.194. and 215. Furthermore, they tend to be centered on the dermis with secondary involvement of the subcutis.
In recent years, attention has been drawn to cases of subcutaneous panniculitis-like T-cell lymphoma that presented with lichenoid (interface) changes, and resembling lupus erythematosus panniculitis. 216 Even mucin has been present in the dermis. 217 Magro and colleagues have expressed the view that a spectrum of subcutaneous T-cell dyscrasias exists which includes lupus profundus, indeterminate lymphocytic lobular panniculitis, and subcutaneous T-cell lymphoma. 218 In a series of 32 cases of lymphocytic lobular panniculitis, 19 were regarded as lupus profundus, six cases were regarded as indeterminate, and seven as subcutaneous T-cell lymphoma. 218 In a later publication they introduced the term atypical lymphocytic lobular panniculitis for 12 patients, prospectively encountered, presenting with a lymphocytic panniculitis accompanied by atypia, but not fulfilling the criteria for subcutaneous T-cell lymphoma.219. and 220. They had an indolent course. The majority of the cases showed clonal T-cell receptor-γ (TCR-γ) rearrangements. 219 An Editorial in response to this article, by McNutt and Fung, stated that this should be considered ‘a provisional diagnosis’, while a letter from Ackerman called this concept ‘humbug on every score’. Suffice it to say that diagnostically difficult cases of lymphocytic lobular panniculitis are sometimes seen. The author has seen several cases with initial lupus-like features that progressed to overt subcutaneous T-cell lymphoma.
Pancreatic panniculitis manifests as painful or asymptomatic subcutaneous nodules or indurated plaques on the thighs, buttocks, lower trunk or distal extremities, usually the lower. The lesions are associated with acute pancreatitis221.222.223.224.225.226. and 227. or, less commonly, pancreatic carcinoma,228. and 229. either of which may be asymptomatic.230.231. and 232. Pancreatic panniculitis has been reported in a 4-year-old child with the nephrotic syndrome. 233 It has also been associated with low-grade pancreatitis in a patient with a pancreas divisum. 234 There may also be polyserositis, arthritis, eosinophilia, or rarely a leukemoid reaction. The pancreatic carcinoma, when present, is often of acinic type, although panniculitis has also been reported in association with an islet cell carcinoma.235.236.237.238. and 239.
Lesions probably result from the local action of blood-borne pancreatic lipase and trypsin, although other factors may also be involved.230. and 240. Cases have been reported without pancreatic disease, but with circulating lipase or amylase of uncertain origin. 241
Sections of established lesions show a lobular panniculitis involving much of the fat of the affected lobule. Sometimes, contiguous lobules show a different stage in the histological evolution of the process. Early lesions show enzymatic fat necrosis, with the ghost-like outline of fat cells remaining (Fig. 17.8). It has been suggested, on the basis of one case, that at an even earlier stage (2-day-old lesions) there is a septal panniculitis. 242 Liquefaction with breakdown of fat cells will eventually occur. At the margins of the necrotic fat there is a variable neutrophil infiltrate, usually mild, associated with nuclear dusting, fine basophilic calcium deposits, and some hemorrhage. 243 The necrotic fat cells may also have a pale basophilic hue due to the deposition of calcium salts. In older lesions there are giant cells, lipophages, lymphocytes, hemosiderin and other blood pigments, and eventual fibrosis. There may be some extension of the inflammatory process into the underlying dermis.
(A) Pancreatic fat necrosis characterized by enzymatic fat necrosis surrounded by a zone containing nuclear dust and an inflammatory cell infiltrate. (B) The fat necrosis has a characteristic appearance. (H & E)
Lupus panniculitis (sometimes referred to as lupus (erythematosus) profundus) is a chronic, recurrent panniculitis with a predilection for the proximal extremities, trunk, or lower back: it is a complication in approximately 1–3% of patients with cutaneous lupus erythematosus, both systemic and discoid forms.244.245.246.247. and 248. Rare sites of involvement in Western populations include the scalp249 and the earlobe. 250 The face and scalp are common sites of involvement in Asians. 251 There is a female predominance. While most cases develop in middle-aged adults, onset in childhood has been reported.252.253.254. and 255. The lesions present as subcutaneous nodules or indurated plaques, although large, painful, indolent ulcers may develop.256. and 257. Lipoatrophy may result. This was accompanied by localized hypertrichosis in one case. 258 The panniculitis may precede, accompany, or follow the development of the associated lupus erythematosus; 247 in nearly half of the cases no associated lupus subtype or autoimmune disease is present.259. and 260. Accordingly, lupus panniculitis should be regarded as a unique entity within the lupus spectrum. 259 It has been suggested that a type I interferon driven immune response may be responsible for the lesions. 261 The lobular panniculitis is dominated by cytotoxic CXCR3+ lymphocytes. 261
A panniculitis mimicking lupus panniculitis has been reported in a case of malignant atrophic papulosis (Degos’ disease); 262 many pathologists now regard malignant atrophic papulosis as being part of the ‘lupus spectrum’. A lupus-like lobular panniculitis may develop at the injection site of glatiramer acetate, used in the treatment of multiple sclerosis. 263
Treatment options include corticosteroids, cyclosporine (ciclosporin), dapsone, and antimalarials such as hydroxychloroquine.255. and 264. Topical treatment with potent corticosteroids may be used for localized lesions.
In up to half the cases there are epidermal and dermal changes of lupus erythematosus (particularly in those complicating discoid lupus erythematosus), with basal vacuolar change and a superficial and deep perivascular lymphocytic infiltrate with perifollicular involvement. The group from Graz, Austria, found dermal infiltrates in 82% of cases and mucin deposition in 73%. 265 There is a lobular panniculitis with a prominent lymphocytic infiltrate (lymphocytic lobular panniculitis).196. and 245. Concomitant septal involvement is often present. 265 A lymphocytic vasculitis with lymphocytic nuclear dust is sometimes present. A characteristic feature, found in 20–50% of cases, is the presence of lymphoid follicles, sometimes with germinal centers, adjacent to the fibrous septa (Fig. 17.9). Lymphoid follicles are uncommon in other panniculitides but they are occasionally seen in morphea, erythema nodosum, and erythema induratum–nodular vasculitis. 266 Plasma cells are present in many cases while eosinophils may be seen in up to 25% of cases. 267 Lipophages may sometimes be quite numerous. Myxoid and hyaline change may be found in the connective tissue septa and lower dermis. Hyaline sclerosis may extend into the lobules. Sometimes there are calcium deposits in older lesions. Membranocystic (lipomembranous) changes may be present.259.268.269. and 270.
Lupus panniculitis. (A) Paraseptal lymphoid follicles are characteristic. (B) Lymphocytes extend into the adjacent fat lobules. (H & E)
The main differential diagnosis is subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Cases of this entity presenting as lupus erythematosus panniculitis have been well documented. The most useful criteria for distinguishing lupus panniculitis from SPTCL are the presence in lupus panniculitis of epidermal involvement, lymphoid follicles with reactive germinal centers, a mixed cell infiltrate with prominent plasma cells, clusters of B lymphocytes, and polyclonal TCR-γ gene rearrangement. 265
Connective tissue panniculitis is extremely rare. 271 It tends to occur in female adults and children. It is characterized by multiple nodules and plaques on the trunk and lower limbs.271. and 272. Low-titer antinuclear antibodies are usually present. Cases followed by lipoatrophy have been included as a variant;273.274.275. and 276. this may be the natural course of the disease. Other autoimmune diseases or a family history of autoimmune disease may be present. 271
The reported cases have shown a lobular panniculitis with some fat necrosis resembling the appearance in erythema induratum–nodular vasculitis but showing no vessel changes and no granulomas. There have been dense lymphocytic infiltrates, rare lymphoid follicles, and small foam cell collections.271. and 272.
A pustular panniculitis has been reported in one patient with rheumatoid arthritis. 277
Poststeroid panniculitis is a rare complication of systemic corticosteroid therapy. It is characterized clinically by the development, usually on the cheeks, of multiple subcutaneous nodules within days or weeks of the withdrawal of steroid therapy.278. and 279. It usually occurs when the steroid is abruptly tapered or stopped. The lesions heal without scarring.
There is a non-specific lobular panniculitis with lymphocytes, histiocytes, and some foam cells and giant cells. Septal vessels are spared, distinguishing the lesions from those of erythema induratum–nodular vasculitis. Needle-shaped clefts, resembling those seen in subcutaneous fat necrosis of the newborn, are present within adipocytes. 279
Subcutaneous sarcoidosis is an uncommon form of sarcoidosis that presents with subcutaneous nodules, principally on the extremities. It constituted 2.2% of the cases of cutaneous sarcoidosis on file at the Mayo Clinic. 280 This entity should not be confused with the erythema nodosum that may be associated with sarcoidosis, particularly in cases of Lofgren syndrome (see p. 460). Sarcoidosis is discussed further in Chapter 7, pp. 171–173).
The granulomas are lobular in distribution. There is usually some peri-granulomatous fibrosis, but this usually spares the septae. While many of the granulomas are so-called ‘naked’ in type, other are more tuberculoid with a rim of lymphocytes. This feature is typical of the subcutaneous form. 281 There may be some necrosis at the center of the granulomas, simulating tuberculosis. 8 Calcification may also be present.
The term ‘lipodystrophy’ has traditionally been used for primary, idiopathic atrophy of subcutaneous tissue, whether total, partial, or localized. 282 It has been distinguished from the secondary lipoatrophy that may follow certain panniculitides such as lupus panniculitis, connective tissue panniculitis, and subcutaneous morphea.274.282.283.284. and 285. Lipoatrophy may be a diagnostically challenging presentation of T-cell lymphoma. 286 The term ‘lipodystrophy’ is also used for the lipoatrophy (and the rare lipohypertrophy) that may follow the repeated injection of insulin into the subcutis by diabetics. 287 The term ‘corticosteroid-induced lipodystrophy’ has been used for the morphological changes that follow the use of corticosteroids. 288 Adipose tissue accumulates in the face (‘moon’ face), the dorsocervical region (‘buffalo hump’), the supraclavicular area, and the abdomen. 288 It has been suggested that lipedema, characterized by the abnormal deposition of subcutaneous fat in the legs (associated with edema), is a lipodystrophy. 289 The three major categories of lipodystrophy will be considered further, followed by a discussion on lipedema.
Total lipodystrophy may be congenital or acquired. Congenital generalized lipodystrophy (Beradinelli–Seip syndrome) is an autosomal recessive disorder with complete loss of adipose tissue at birth, with the exception of the adipose tissue of the orbits, palms, soles, vulva, breast, and tongue. 290 There are at least three types: type 1 (OMIM 608594), which is caused by a mutation in the gene encoding 1-acylglycerol-3-phosphate O-acyltransferase-2 (AGPAT2) on chromosome 9q34.3; 291 type 2 (OMIM 269700), which is caused by a mutation in the gene encoding seipin (BSCL2) located at 11q13; 292 and a third locus, caveolin-1 (CAV1), which has recently been identified in an adolescent female. 293 The genetic basis of a fourth novel subtype remains to be determined. 293
Partial lipodystrophy begins usually with symmetrical loss of facial fat, often progressing to involve the upper trunk and arms. 295 It may be familial or acquired. The familial variant (Dunnigan type) is discussed after the acquired type, which is more common. Two variants of acquired partial lipodystrophy (Barraquer–Simons disease) have been described. In the Weir–Mitchell type there is loss of fat from the face, with or without atrophy of the arms and upper trunk, while in the Laignel–Lavastine type there is concomitant hypertrophy of the fat of the lower part of the body. 296 Unilateral variants of this have been described;297. and 298. facial hemiatrophy is known as the Parry–Romberg syndrome.297. and 299. Cases of partial lipodystrophy have been related to thyroid disease, extrinsic allergic alveolitis, 300 acanthosis nigricans, dermatomyositis,301. and 302. myasthenia gravis, the antiphospholipid syndrome, 303 membranoproliferative glomerulonephritis,304.305. and 306. hepatitis B infection, 307 and recurrent infections. 246 Infundibulocystic proliferations occurred on the finger in one case. 308 Recently, an acquired lipodystrophy was reported in a 3-year-old girl in whom additional material was detected on chromosome 10 at the 10q26 location, at the site of the human pancreatic lipase gene. 309 However, most cases of acquired partial lipodystrophy probably have an immunological mechanism.
Familial partial lipodystrophy (Dunnigan type, OMIM 151660) is an autosomal dominant disorder with normal adipose distribution in childhood. Gradual loss of fat from the extremities and trunk, and increased fat deposition on the face and neck begin at puberty. 290 It is one of the nine laminopathies caused by mutations in the LMNA gene that encodes the nuclear envelope proteins lamin A and lamin C. 310 The gene has been mapped to chromosome 1q21–q22. Other disorders of this gene, discussed in this book, result in progeria of Hutchinson–Gilford type (see p. 329) and restrictive dermopathy (see p. 324).
Localized lipodystrophy is characterized by annular or semicircular areas of atrophy, often solitary. It needs to be distinguished from morphea-related lipoatrophy. 311 Variants have been described near the ankles,184.312.313. and 314. on the thighs,185.315. and 316. over the sacrum, 317 on the abdomen (lipodystrophia centrifugalis abdominalis),318.319.320.321.322.323.324.325.326. and 327. and in the form of a unilateral linear panatrophy of the extremities. 328 In a series of 30 Korean patients with lipodystrophia centrifugalis abdominalis, 4 were adult women, 20 were girls, and 6 were boys. 327 The mean age of onset was 6.2 years. White people are uncommonly affected. 329‘Post-injection’ lipoatrophy is another variant of localized lipodystrophy. It can follow the injection of corticosteroids, 330 methotrexate, 316 iron dextran, vaccines, 331 insulin, and penicillin.332.333. and 334. The injection of glatiramer acetate for the treatment of multiple sclerosis produces a lobular panniculitis that may resemble lupus panniculitis. 263 In one series of localized lipoatrophy, 9 of the 16 patients had received injections at the site. 335 Pressure and microtraumas appear to be involved in the causation of some other cases of the localized variant, particularly semicircular lipoatrophy.315.336.337.338. and 339. Leg crossing is another cause of dimpling and localized lipoatrophy. It produces pressure in the area that rests on the patella of the opposing knee. 340 Hyperpigmented circumferential bands may develop as a consequence of wearing tight socks. There is no evidence that lipoatrophy is present in these cases. 341 Linear atrophic patches have been reported as a manifestation of the complex regional pain syndrome. 342 Apoptosis of fat cells has been noted during the lipoatrophic process in one case of abdominal lipodystrophy. 343
Lipedema is characterized by diffuse, bilaterally symmetrical, enlargement of the buttocks and legs due to the subcutaneous deposition of fat. 344 The feet are much less involved, or spared entirely. 345 There is some clinical resemblance to lymphedema. Women are almost exclusively involved, the lipedema typically developing insidiously after puberty. 345 Approximately 10% of women may be affected to some degree. It occurs independently of lymphatic or venous insufficiency.
The histopathology of the three major categories of lipodystrophy and that of lipedema will be considered together.
In established cases there is atrophy of subcutaneous fat and usually no evidence of inflammation. In some cases, biopsy of an early lesion shows a lobular panniculitis, often with some vascular involvement.346.347.348.349. and 350. In one personally studied case there was a lobular panniculitis with numerous foam cells, small fat cysts, and some lymphocytes; there was no vasculitis (Fig. 17.10). This patient had partial lipodystrophy with prominent facial involvement and a poorly characterized connective tissue disease. Her inflammatory lesions were transient.
(A) Partial lipodystrophy at the stage of a lobular panniculitis. Atrophy developed subsequently. (B) Inflammatory changes are usually mild. (H & E)
It has been suggested that there are two histopathological types of lipodystrophy but whether this is always stage-related (see above) has not been clarified, as yet. 346 In one group there are prominent involutional changes to the subcutaneous fat with small lipocytes and intervening hyaline or myxoid connective tissue containing numerous capillaries. The second group has inflammatory changes characterized by a lobular panniculitis with lymphocytes, lipophages, and plasma cells. This pattern was present in the young girl with abnormalities of chromosome 10 (see above). A dermal lymphocytic vasculitis has also been described. 296 The inflammatory type usually has multiple areas of lipoatrophy clinically and immunoreactants in blood vessels or the basement membrane on direct immunofluorescence.
The localized variants, such as semicircular and post-injection lipoatrophy, are infrequently biopsied. They show loss of fat with diminutive fat lobules within a partially fibrosed stroma. 330 There is usually no evidence of panniculitis, although inflammation has been reported. 351 Striking perieccrine inflammation of lymphocytic type was present in one case. 352 Occasional macrophages may be seen in close proximity to lipocytes. They appear to engulf segments of altered adipose tissue and stroma. 330 There are often scattered lipophages.335. and 336. Epidermal atrophy and dermal fibrosis sometimes accompany the changes in the fat. 353 The appearances can resemble embryonic fat. Most cases correspond to the first group mentioned above.
In lipedema there is marked circumferential enlargement of the subcutaneous tissue due to fatty hypertrophy. Fat lobules are surrounded by thickened fibrous septa. There may be some edema and adipocyte degeneration. 344 Pseudoxanthoma-like changes were present in the subcutis in one case. 344
A lipodystrophy can be seen in patients with the acquired immunodeficiency syndrome (AIDS). Three clinical settings appear to be involved. The most common variant, characterized by the presence of peripheral lipoatrophy and central adiposity, can develop following the use of protease inhibitor therapy.354.355.356.357. and 358. Clinically evident lipodystrophy usually begins 2–14 months after the commencement of therapy. The changes include loss of fat from the arms, buttocks, thighs, and legs, leading to prominence of veins and muscles, particularly on the legs. 359 Loss of the buccal fat pad gives a cachectic appearance. 356 The accompanying lipohypertrophy includes central obesity, enlargement of the dorsocervical fat pad (‘buffalo hump’), and deposition of abdominal fat. Indinavir is the protease inhibitor most often implicated. Resolution of the lipodystrophy usually occurs on discontinuation of the therapy. 354 It has been suggested that protease inhibitors bind to proteins that regulate lipid metabolism. Dysregulation of peroxisome function also occurs. 360
A similar lipodystrophy has been reported with the use of reverse transcriptase inhibitors. It has been suggested that these cases may in some way be related to suppression of HIV replication. 357
Finally, a third group of HIV-related cases appears to exist. Some patients with AIDS develop a facial lipoatrophy; not all have been on protease or reverse transcriptase inhibitor therapy.
Autologous fat grafts for the enhancement of facial contours have met with high patient satisfaction rates. 361 Polylactic acid implants are another successful strategy. 362
The lipoatrophy resembles the non-inflammatory variant of lipodystrophy (see above). There is atrophy of fat, sometimes accompanied by an alteration in the size and shape of adipocytes. The fat cells were reported as having a normal spherical shape in one report. 357 Focal collections of lymphocytes and lipophages are sometimes present, but they are not a prominent feature. A proliferation of small vessels may occur at the interface between the atrophic lobules and the septa, resembling immature (fetal) fat.
Gynoid lipodystrophy, better known as cellulite, is an alteration of the topography of the skin that occurs mainly in women on the buttocks, thighs, and abdomen. 363 It gives the skin a dimpled (‘orange peel’, ‘quilted’, ‘mattress’) appearance.364. and 365. It is very common in obese patients, although it differs from obesity both structurally and mechanistically.363. and 366. Four grades of clinicopathological severity have been defined. 363
The pathogenesis of gynoid lipodystrophy is complex and still being elucidated but it includes alterations in the composition and the amount of fat in adipocytes, changes in the microcirculation of the subcutis, and hyperpolymerization in the connective tissue of the fat septa. 363 These changes appear to be influenced by hormonal and psychosomatic factors as well as by race, sedentary lifestyle, and an inherent predisposition. 363 Cellulite appears to predispose to earlier skin aging changes. 367
There is no consensus on the histopathological features of gynoid lipodystrophy, possibly reflecting different stages of the disease process. Furthermore, there appears to be a physiological difference between the dermal–subcutaneous interface in men and women, with a smooth interface in males and a tendency to a ‘lumpy’ appearance in females as a consequence of the protrusion of superficial fat lobules into the dermis. 364
In early stages, the superficial fat lobules are large. They may be squeezed between straightened fibrous septa, which are of variable thickness. This latter feature becomes more obvious in established cases when lumpy and loose swellings are interposed between thinner areas of the septa; a few myofibroblasts are present. The septa may come to resemble areas of striae distensae (see p. 319) with associated alterations in the elastic tissue which may consist of clumped and increased fibers and other areas with reduced elastic tissue. 364 Small collections of adipocytes may become encapsulated with fibrous strands.
Vascular changes are variable and may involve the dermis as well. There is often neovascularization, dilatation of vessels, intimal thickening of small arteries, and microaneurysms with hemorrhage. 363
The term ‘membranous lipodystrophy’ was originally applied to a sudanophilic sclerosing leukoencephalopathy in which cystic degeneration of the marrow of long bones also occurred. 368 The fat showed a characteristic lipomembranous (membranocystic) change (see below). The term has since been applied to a varied group of panniculitides in which this same lipomembranous change occurs. The term pseudomembranous fat necrosis is also used. 369 There are several cases in the literature where this change has been found as a primary phenomenon in the subcutaneous fat of the skin; it has therefore been proposed that such cases should be designated primary membranous lipodystrophy to distinguish them from the cases in which it is merely an incidental histological feature – secondary membranous lipodystrophy.370.371. and 372. Diseases in this latter category include erythema nodosum,269. and 373. morphea profunda, 368 lupus panniculitis, traumatic fat necrosis, insulin lipoatrophy, 374 the panniculitis of Behçet’s syndrome, encapsulated fat necrosis, and the panniculitis of dermatomyositis.369. and 375. Membranous lipodystrophy also occurs in association with circulatory disturbances, particularly diabetic microangiopathy and venous insufficiency; vasculitis-induced lesions are rare. 376 As lipodermatosclerosis also occurs in a setting of venous insufficiency, it is perhaps not surprising that lipomembranous change is common in lipodermatosclerosis. 377
It seems likely that membranous lipodystrophy will eventually be regarded as synonymous with lipomembranous (membranocystic) change – a histological change rather than a disease sui generis.
Membranous lipodystrophy (pseudomembranous fat necrosis) is characterized by lipomembranous (membranocystic) change. There are cysts of varying size, usually small, lined by amorphous, eosinophilic material having an arabesque architecture (Fig. 17.11). It results from the interaction of residual products of disintegrated fat cells and macrophages. 369 Sometimes the membranes form small pseudopapillae. 378 The material is stained by the PAS and Sudan black methods. Between groups of fat cysts there are dense, thick, fibrous septa. Myospherulosis (see p. 396) has been noted in two cases.379. and 380. In secondary membranous lipodystrophy, changes of the underlying process will, of course, be present as well.
(A) Lipomembranous fat necrosis. (B) There is some sclerosis in the wall of the fat cyst which is lined by hyaline eosinophilic material with an arabesque pattern. (H & E)
Criticisms notwithstanding, 381‘lipodermatosclerosis’ is still the preferred term for an entity characterized by circumscribed, indurated, often inflammatory plaques on the lower extremities. In a series of 97 patients reviewed at the Mayo Clinic, 84 were women, and the mean age was 62 years. 382 Lesions were bilateral in nearly half of the cases. The condition is usually associated with venous insufficiency, arterial ischemia, or previous thrombophlebitis.259. and 383. One case developed during therapy with gemcitabine, 384 and another as a form of radiation recall dermatitis. 385 It may also complicate chronic lymphedema. There may also be mottled hyperpigmentation related to venous stasis. 386 Sometimes the clinical appearances mimic a cellulitis. Similar lesions have been reported as sclerosing panniculitis387 and hypodermitis sclerodermiformis. So-called ‘lipomembranous’ (membranocystic) change is frequently seen on histology, thus this condition has also been regarded as a membranous lipodystrophy (see above) or simply called lipomembranous (membranocystic) fat necrosis. 269
Lipodermatosclerosis appears to be a consequence of ischemia and venous stasis. Venous insufficiency was thought to be present in a case in a 15-year-old girl. 388 There is abnormally low plasma fibrinolytic activity in these patients, as well as low levels of proteins C and S. 389 However, fibrinolytic activity is increased in affected tissues and this is associated with elevated levels of urokinase-type plasminogen activator (uPA). 390 In addition, there is elevated matrix turnover, although the significance of this finding is uncertain. 391 Sclerosis is also a feature. 392 There is a significant increase in the number of dermal fibroblasts undergoing proliferation, and an increase in the number of cells expressing procollagen type 1 mRNA. 393 Ultrasound investigations have shown that the edema fluid in this condition is predominantly in the upper dermis in contrast to the deep dermal location of the fluid in chronic heart failure. 394 Magnetic resonance imaging (MRI) can be used as a diagnostic tool in this condition. 395 As light compression appears to enhance the removal of edema fluid, compression stockings have been used in the treatment of lipodermatosclerosis. 396 Other therapies used have included stanozolol combined with compression therapy, surgical correction of venous disease, pentoxifylline, and intralesional triamcinolone. 397
Biopsies are usually obtained in the later stages of the disease when there is septal fibrosis and sclerosis and fatty microcysts with foci of membranocystic change. This consists of amorphous eosinophilic material, sometimes with a crenelated appearance, lining microcysts. This material is PAS positive and stains with Sudan black. 370 Microgranules may be present in macrophages. Hemosiderin pigment is often present in the dermis; it may also be in the subcutis. There is fibrosis of the dermis and pericapillary fibrin caps around small vessels in the upper dermis. The fibrous thickening of the lower dermis means that a punch biopsy of an involved area of skin may not include any subcutaneous fat (Fig. 17.12).
(A) Lipodermatosclerosis. (B) Fibrous tissue replaces the subcutis. There is mild chronic inflammation at the level of the sweat glands. There is some hemosiderin pigment in the lower dermis. (H & E)
In early lesions (uncommonly biopsied as the site heals poorly), there is a septal and lobular panniculitis, with lymphocytes being the predominant cell. There is variable fat necrosis; sometimes an entire lobule is infarcted. Blood vessels appear prominent in the septa at all stages.
The clinical manifestations of self-induced panniculitis will depend on the nature and site of the insult. Factitial panniculitis may follow injections of all manner of substances, including milk, urine, feces, oils (oleoma), and drugs, into the subcutaneous fat. Reports have included the injection of procaine povidone, 398 meperidine (pethidine), 399 sesame oil, 400 and pentazocine. 401 A case masquerading as pyoderma gangrenosum has been reported. 402 Sclerosing lipogranuloma (paraffinoma) is a special form of factitial panniculitis resulting from the injection of lipid, often paraffin, into the subcutaneous tissue, 403 particularly in an attempt to produce enlargement of the penis and even the breasts.404. and 405. Other oils and silicones have been involved.406.407. and 408. The lesion presents as a painful rubbery induration of the involved area. Fistulas and ulceration are common. 409 Oil cysts developed after the regular subcutaneous injections of interferon-α and interleukin-2 for metastatic melanoma. However, as the injectable solutions are water based, it was postulated that the injection of these cytokines may have led to a focal liponecrosis with resulting fat cysts. 410 Mesotherapy injections for ‘cellulite’ produced a granulomatous panniculitis with some fat cysts. 411
A localized panniculitis has also been reported following the subcutaneous injection of glatiramer acetate in the treatment of multiple sclerosis.263. and 412. Transient reactions and pain are not uncommon complications, but more persistent lesions occur.
Although factitial panniculitis usually produces a lobular or mixed panniculitis, other patterns such as a suppurative septal panniculitis can occur (Fig. 17.13). 402 Foreign body giant cells with foreign material, sometimes doubly refractile, may be present. It is a good idea to examine by polarized light any unusual suppurative panniculitis or one with foreign body-type granulomas.
(A) An unusual case of factitial panniculitis. (B) There is suppuration involving the interlobular septa. The deep fascia contains some doubly-refractile foreign material, the nature of which was not ascertained. (H & E)
In paraffinomas and oleomas, there is a characteristic Swiss-cheese appearance with disruption of fat cells and their replacement by cystic spaces of variable size, some surrounded by attenuated foreign body giant cells containing lipid vacuoles.405. and 408. There are bands of hyaline fibrous tissue between the fat cysts. The septa contain a scattering of lymphocytes and lipid-containing macrophages and foreign body giant cells. This pattern is referred to as sclerosing lipogranuloma.
The reactions that developed following the injection of glatiramer acetate consisted of a mostly lobular panniculitis, with histiocytes and T lymphocytes in the fat lobules, and thickened septa with scattered lymphoid follicles. 412
An unusual pattern of transdermal elimination of fat cells occurs after fat necrosis of various etiologies. The author has seen it follow erythema nodosum, traumatic fat necrosis, and lupus erythematosus panniculitis. Clinically, the lesion may weep over an area of induration. Clusters of degenerated fat cells are seen high in the dermis. Small tissue spaces may represent degenerate fat removed during tissue processing.
The term pseudosclerodermatous panniculitis was used by Winkelmann and colleagues in 1993 to describe four cases of women with breast cancers who received megavoltage radiotherapy. 413 Further cases have since been described. 414 All patients developed progressive induration of the subcutaneous tissues in the irradiated area. In one series, the interval between radiotherapy and the development of the induration varied from 4 to 8 months. 414
The main findings were confined to the subcutaneous tissue and consisted of thickened, sclerotic septa combined with a lobular panniculitis characterized by lipophagic granulomas, lipophages, and scattered lymphocytes and plasma cells. 414
Subcutaneous fat necrosis may follow trauma, particularly to the shins. Focal liquefaction of the injured fat often follows and this is sometimes discharged through a surface wound. Some cases of traumatic fat necrosis have a factitial origin, so there is obvious overlap with factitial panniculitis. 415
Cases coming to biopsy usually show fat cysts of varying size with surrounding fibrosis. The microcysts sometimes show lipomembranous (membranocystic) change. There are often small collections of foam cells, a mild patchy lymphocytic infiltrate, and some hemosiderin. In earlier lesions there is fat necrosis with cystic spaces and numerous neutrophils in the adjacent fat. A similar appearance follows surgical disruption of the subcutis. 416 Sometimes collections of fat cells, often necrotic, can be seen within the dermis, apparently in the process of being eliminated through a break in the epidermis.
Encapsulated fat necrosis is the preferred designation for the small mobile nodules that may develop in the subcutis; they often follow trauma.417. and 418. These lesions have also been called ‘mobile encapsulated lipoma’ and ‘nodular-cystic fat necrosis’. 419
The lesions, which are usually solitary, are whitish-yellow in color, and measure 3–20 mm in diameter. 417 An unusually large case, measuring 18 cm in longest diameter, has been reported in an elderly female with morbid obesity. 420 Encapsulated fat necrosis has also been reported in a patient with sarcoidosis. 421
The lesions are composed of lobules of necrotic fat surrounded by a thin fibrous capsule which is usually hyaline (Fig. 17.14). Lipomembranous change (see above) is often present.371.420. and 422. Small collections of lipophages and very focal inflammatory changes are sometimes seen, particularly near the capsule. Dystrophic calcification may be a late complication.
Encapsulated fat necrosis. There is a hyaline capsule with central fat necrosis and cystic change. (H & E)
Various infections and infestations may result in a lobular or mixed lobular and septal panniculitis.4. and 423. These include candidiasis, 370 cryptococcosis, mycetoma, actinomycosis, nocardiosis, chromomycosis, sporotrichosis, and histoplasmosis,424. and 425. infections with Mycobacterium marinum, 426M. chelonae, 427M. ulcerans, M. fortuitum, M. tuberculosis, 428M. leprae (erythema nodosum leprosum – see p. 566), M. avium-intracellulare, 429Neisseria menigitidis and other bacteria,423. and 430. bites of ticks, 431 and of the brown recluse spider (Loxosceles reclusa), 51 and metazoal infestations (myiasis, cysticercosis, sparganosis, and infestations by some other helminths). Most cases of infection-induced panniculitis occur in patients who are immunosuppressed. 423 Two subcutaneous nodules developed in a patient with chronic myeloid leukemia who contracted Q fever. 432
The presence of a lobular or mixed lobular and septal panniculitis in which there is a heavy infiltrate of neutrophils, 428 often with extension into the dermis, should raise the suspicion of an infective etiology (Fig. 17.15). Hemorrhage and necrosis are often present. In a case of neutrophilic lobular panniculitis due to acanthamebiasis, trophozoites measuring 20–30 µm in diameter were present. 433
Mycobacterium ulcerans. The presence of a widespread suppurative panniculitis, as shown here, warrants exclusion of an infective etiology. Numerous acid-fast bacilli were present. (H & E)
The patient who had Q fever presented with a granulomatous lobular panniculitis with a characteristic ‘fibrin ring’ or ‘doughnut’ appearance with fibrin and inflammatory cells arranged around a central clear space. Similar changes have been described in the liver and bone narrow in this disease. 432
Neutrophilic panniculitis presents with multiple, often painful, subcutaneous nodules on the legs, arms, and trunk. Between 10 and 20 nodules may be present. 434 The lesions are erythematous and deep on palpation. They tend to last about 15 days. 434 While an infective cause should always be considered in the presence of a neutrophilic panniculitis, other etiologies do exist.435. and 436. In the early stages of many of the panniculitides, neutrophilic infiltration occurs. Examples include erythema nodosum, Behçet’s disease, pancreatic panniculitis, and factitial panniculitis. Other causes of neutrophilic panniculitis include rheumatoid arthritis, 435 including the juvenile form, 437 Sweet’s syndrome,438. and 439. and Crohn’s disease. 440 Myelodysplastic syndromes441 seem to be significantly associated with a neutrophilic panniculitis. A neutrophilic lobular panniculitis has been reported in a patient with acute promyelocytic leukemia treated with all-trans-retinoic acid. 442






