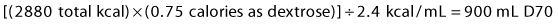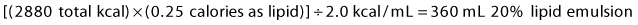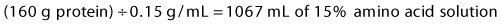Chapter 29 Nutritional support of the burned patient
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
Nutritional therapy is more successful when it is provided within a comprehensive protocol.1–3 Every burn center should develop a multidisciplinary protocol that standardizes the initial and ongoing assessment, initiation, and monitoring of nutritional support for patients with all sizes of burn injury.
A number of recent publications have attempted to summarize the disparate and voluminous literature on nutrition into guidelines for care of critically ill patients;1,4–6 specific guidelines for burn patients have also been published.7,8 These guidelines often fail to agree on the details of nutritional support, which further illustrates our imperfect knowledge of this complicated field. Nutritional support, like many areas in burn care, is a moving target. The reader will need to interpret this chapter, and future readings, in this light.
The hypermetabolism of burn injury
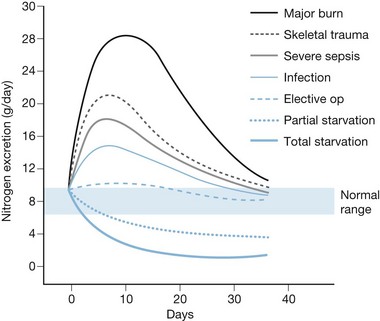
Over 70 years ago, Cuthbertson documented that traumatic injury produced increased energy utilization and accelerated losses of skeletal muscle.9 In the 1970s, burn patients were found to exhibit the most severe hypermetabolism of any group, with energy expenditure from 60% to 100% above normal following a major burn, and concomitant catabolism of protein stores.10,11 These studies also demonstrated the so-called ‘ebb and flow’ response to injury (Fig. 29.1), in which an initial (12–24 h) reduction in metabolic rate is followed by a crescendo–decrescendo curve of sustained hypermetabolism that can persist for weeks.
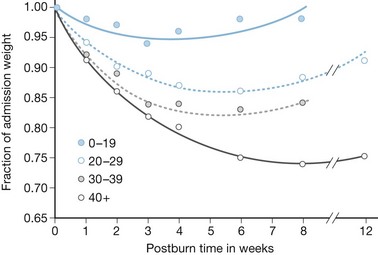
Early attempts to nourish burn patients with oral diets often failed due to altered mental status, gastrointestinal dysfunction, and inhalation injury. Even patients who could eat were rarely able to tolerate the amount of nutrition necessary for adequate support. As a consequence, patients with major burns predictably incurred weight loses of 20% or more within the first few weeks of injury,12,13, with associated immune compromise and delayed wound healing. This inanition often proved fatal, as patients succumbed to respiratory failure, pneumonia, and systemic infection13 (Fig. 29.2).
Mediators of hypermetabolism
The hypermetabolism of burn injury is the product of several predominant hormonal changes.10,11,14,15 Burn injury causes marked and sustained elevations of the catabolic hormones epinephrine, cortisol and glucagon.16 In contrast to unstressed starvation – in which metabolic rate falls, lipolysis and ketosis provide energy, and muscle reserves are protected – this hormonal response to burn injury causes greatly increased metabolism and accelerated gluconeogenesis and glycogenolysis. Catabolic hormones oppose the effects of insulin; as a result, blood sugar levels rise, and protein synthesis and lipogenesis are inhibited.
In this environment, protein breakdown becomes an obligatory major source of energy. Lipids have limited ‘protein-sparing’ effect. In this setting, even glucose is limited in its ability to prevent protein wasting.17 As a result, diets must also be very high in protein to help replace lost lean body mass.
Although these hormonal changes provide a major challenge to the provision of adequate nutrition, they also provide mechanisms by which hypermetabolism can be reduced to facilitate metabolic support. In recent years, investigators have attempted to ameliorate burn-induced hypermetabolism by blocking catecholamines with propranolol, administering the counter-regulatory agents such as insulin and insulin-like growth factor-1 (IGF-1), or using anabolic hormones such as growth hormone, testosterone, or the synthetic anabolic agent oxandrolone.18,19 This rapidly evolving area of research is discussed in detail in other chapters. Although some of these methods show promise for clinical use, it must be remembered that the hormonal milieu that accompanies burn injury is complex and incompletely understood. Additional research will be needed to confirm the efficacy and safety of these techniques.
Modern burn care and metabolic requirements
Modern methods of burn treatment have not altered the nature of burn-induced hypermetabolism but have significantly reduced its magnitude. Maneuvers as simple as maintaining high ambient temperature and relative humidity can reduce caloric requirements by up to 20%.11 Although burn excision has not been shown to reduce energy expenditure directly,20 removing the burn wound and covering wounds with autograft, allograft, or synthetic substitutes removes the inflammatory stimulus of the burn wound, reduces infection, and shortens the duration of hypermetabolism. Other therapies, including mechanical ventilation and chemical sedation/paralysis, also reduce energy requirements.21
As a result, numerous recent reports using indirect calorimetry document metabolic rates which, although still elevated, are now more likely to approximate 120–150% of normal, rather than the 160–200% previously reported.22,23 Nonetheless, both temporal and patient-specific variations in energy expenditure make it difficult to predict requirements for individual patients.
Assessment of nutritional needs
Initial assessment
Pre-existing malnutrition can also exist in burn patients,24 but extensive nutritional assessments are rarely helpful for several reasons. First, burn injury induces major abnormalities in nutritional indices which confound assessment of pre-burn status: swelling and eschar preclude accurate anthropometric measurements, serum proteins are rapidly altered, and immune function is similarly disturbed. A careful nutritional history and functional status may be the most meaningful assessments to perform.25 Moreover, satisfying ongoing requirements is far more important than compensating for pre-existing deficiencies. Attempting to ‘catch up’ by providing extra calories and/or protein is ineffective and likely to increase complications of overfeeding, described below. Therefore, the primary goal of nutritional support in burn patients is to satisfy ongoing burn-specific requirements.
Formulas for estimating caloric requirements
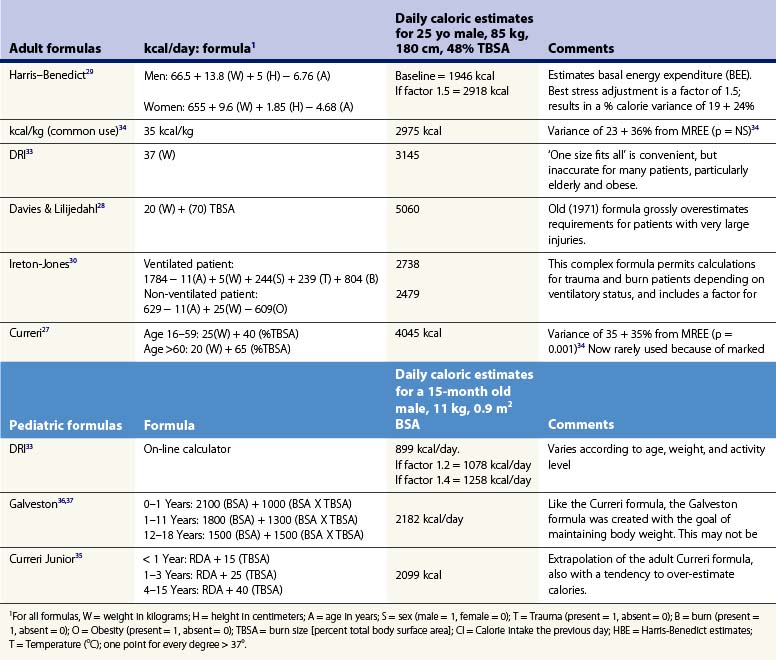
Any protocol for nourishing burn patients must begin with estimation of their nutritional needs. An array of formulas have been developed for this purpose.26 One early example, the ‘Curreri formula,’27 has been widely used, though its accuracy is open to question. To create the formula, Curreri’s group examined only nine patients and calculated backwards to estimate the calories that would have been needed to make up for the patients’ lost weight. As will be discussed, this is a dubious assumption at best.
Several popular formulas for adult nutrition are reviewed in Table 29.1.27–32 These include the ‘dietary reference index’ (DRI) values for normal people developed by the US Department of Agriculture.33 The formulas in Table 29.1 use different variables to predict caloric requirements for specific patients, and reach very different estimates of energy needs. Many older formulas such as Curreri’s significantly overestimate modern requirements,22 and none can account for the major differences in energy expenditure found between patients. Dickerson et al. reviewed 46 formulas for predicting the caloric needs of burn patients.34 They found that none of the methods reviewed correlated accurately (±15% error) with measured energy expenditure in a group of 24 patients. Because post-burn energy expenditure fluctuates dramatically over the course of treatment (see Fig. 29.1), static formulas often result in overfeeding early and late in the post-burn course and underfeeding during periods of peak energy utilization.
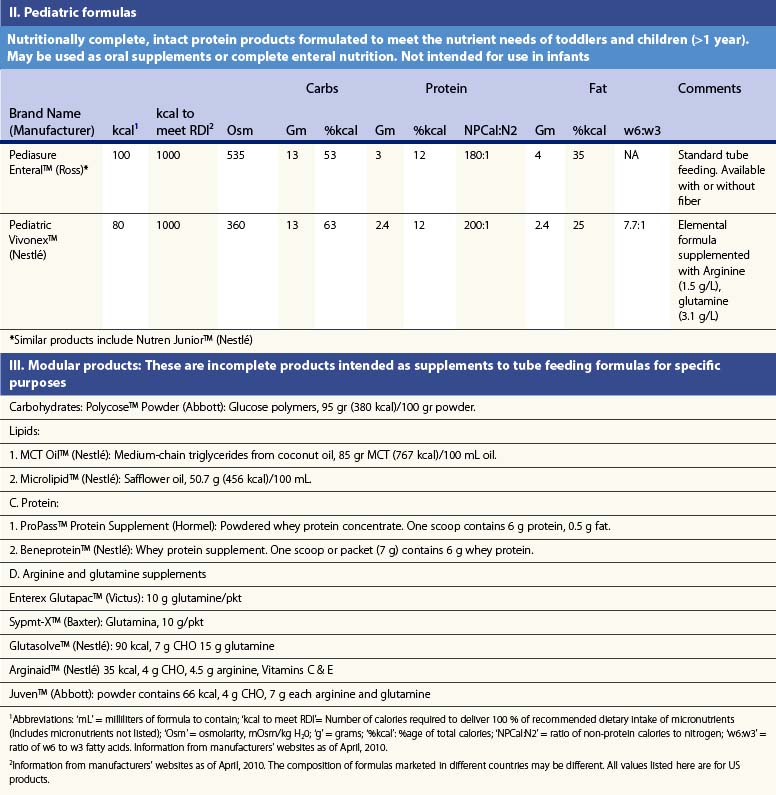
Pediatric formulas
Children present even greater nutritional challenges than adults. Children have lower tolerance for both under- and overfeeding. In addition, requirements change significantly with age, so that different formulas must be used for different age groups. Several commonly used formulas for burned children are also presented in Table 29.1.35–38 These formulas share the same inherent limitations as adult formulas.
Indirect calorimetry
In recent decades, improved technology for performance of indirect calorimetry (IC) has permitted routine measurement of energy expenditure at the bedside. IC devices measure the volume of expired gas and the inhaled and exhaled concentrations of oxygen and carbon dioxide, permitting calculation of oxygen consumption (VO2) and carbon dioxide production (VCO2), and hence metabolic rate.39 Measurements made through tight-fitting face masks, hoods, or by connection to mechanical ventilators have proved reliable and reproducible over a wide range of metabolic rates and FiO2.
Measurement of resting energy expenditure (REE) by IC is usually performed in the early morning with patients at bed rest. Fluctuations in energy utilization associated with activity are usually estimated by increasing REE by 10–20%.40 IC can also detect significant under- or overfeeding through calculation of the respiratory quotient (RQ) – the ratio of carbon dioxide produced to oxygen consumed (VCO2/VO2).41 The body’s metabolism of specific substrates affects this ratio, providing information about metabolic supply and demand. For example, in unstressed starvation, utilization of fat as a major energy source produces an RQ of 0.7 or less;42 in normal metabolism of mixed substrates, RQ = 0.75 – 0.90. In contrast, the synthesis of fat from carbohydrate, which typifies overfeeding, results in an RQ of 1.0 or greater. In this way, overfeeding can contribute to difficulty weaning from ventilatory support.43
Delivering estimated needs: How close is ‘close enough’?
Despite the inherent limitations of static formulas for estimating energy requirements, the superiority of IC has not been proven clinically.44 It is unknown how closely nutritional support must be ‘matched’ to energy requirements; regular episodes of over- and underfeeding are frequent, normal, and often unavoidable. Saffle et al. found no differences in outcomes between patients fed according to the Curreri formula and those whose nutrition was guided by IC.45 Recent studies in ICU patients found that <80% of prescribed calories were actually delivered.46,47 Enteral nutrition was less successful than TPN because of more frequent interruptions for diarrhea, tube dislodgement, surgery, etc. In both types of nutrition hyperglycemia sometimes required feeding to be reduced, whereas dextrose-containing IV fluids and other infusions, such as propofol, added unplanned ‘empty’ calories. These studies illustrate the ubiquitous practical difficulties of actually giving patients the nutrition that is prescribed for them. Abundant experience demonstrates that either formulas or IC can be used to nourish burn patients successfully, particularly if this is done within a multidisciplinary regimen of support. Many units use a goal of delivering nutrition within 10% of measured (or calculated) needs as a quality assurance indicator, despite the lack of evidence for such a practice.
Specific nutrient requirements and ‘pharmaconutrition’
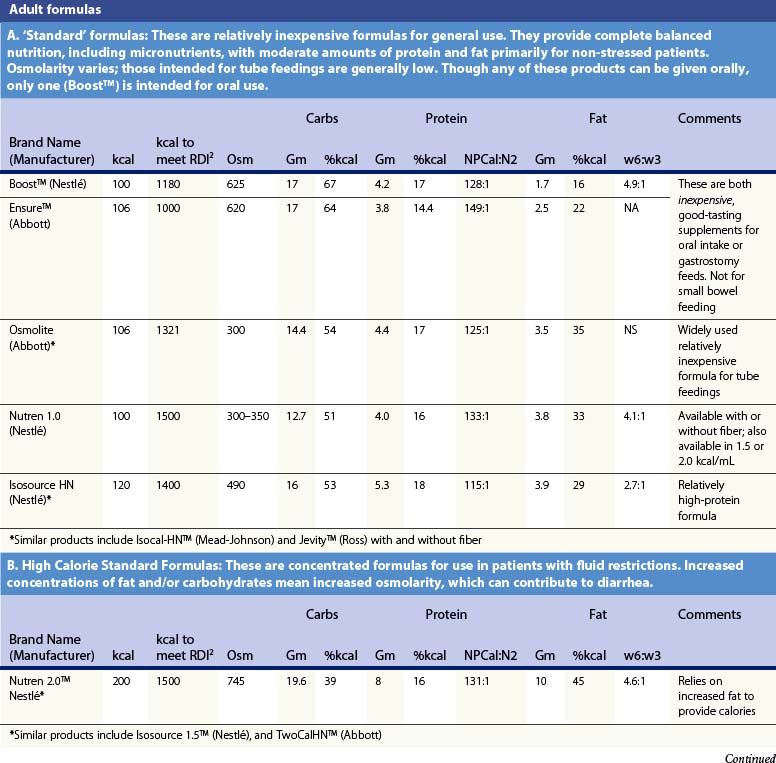
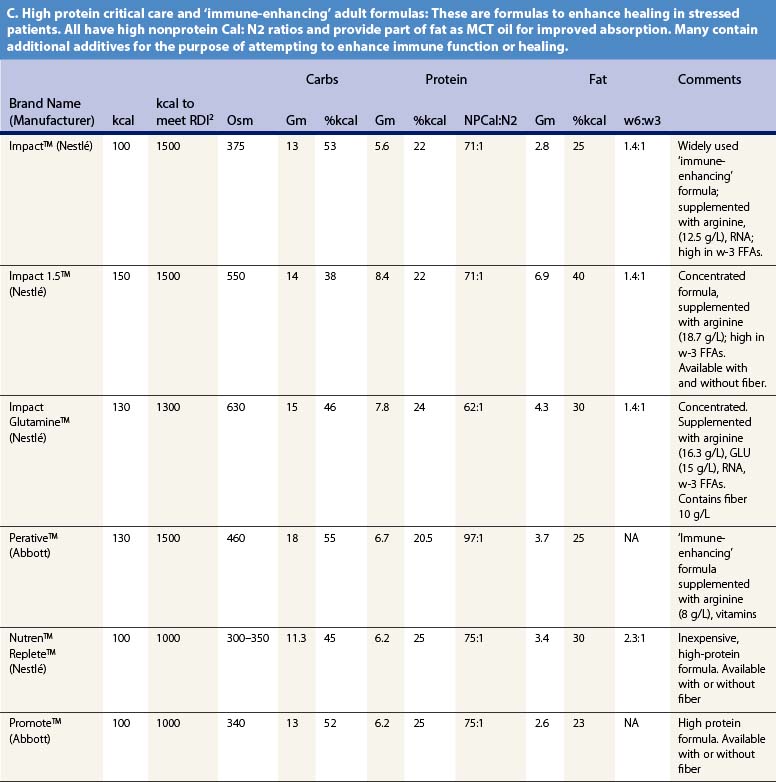
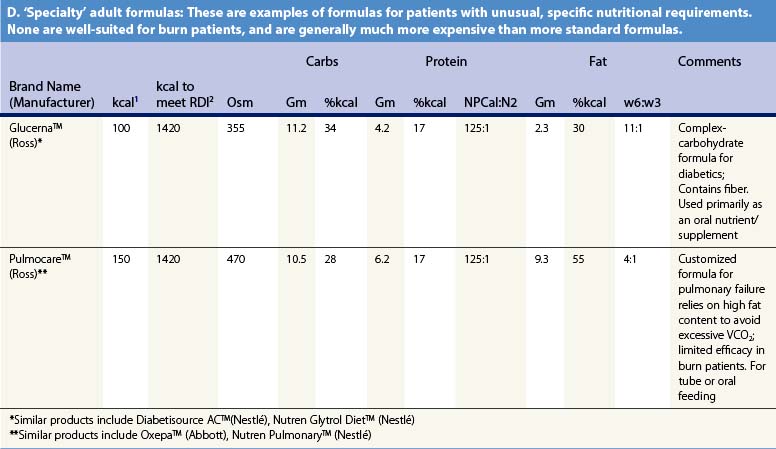
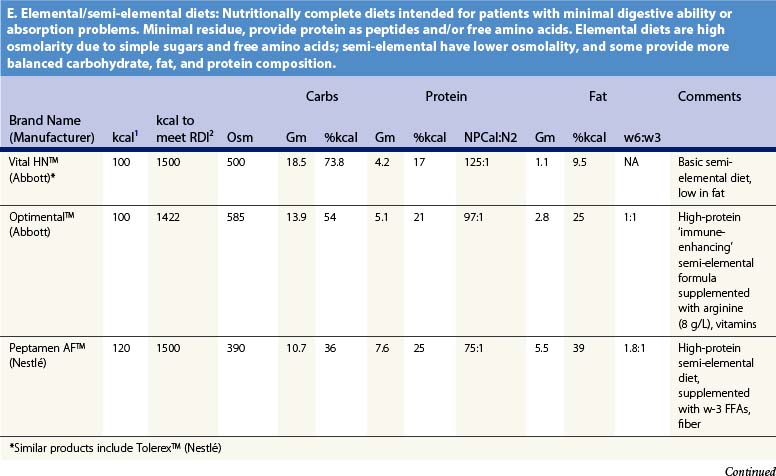
Recent information about the metabolism of specific nutrients has stimulated trials and recommendations for creating disease-specific regimens for patients in a variety of situations – including burns – by changing the basic composition of formulas, and/or providing additives with specific effects on immune function, inflammation, or wound healing, a concept termed ‘immunonutrition,’ and recently further refined as ‘pharmaconutrition.’48 This information, and some of the evidence that supports it (often incomplete), will be reviewed here. Table 29.2, discussed below, lists the composition of a number of commonly available enteral nutrition products which should help in understanding the quantities of nutritients required for support.
Table 29.2 Composition of a sampling of commercially available adult and pediatric enteral nutrition products (note that all values are per 100 ml of feedings)
Requirements and uses of fat
A limited quantity of fat is a required nutrient. Essential fatty acid deficiency is a well-documented complication of long-term TPN;14 for this reason modern parenteral formulas contain significant amounts of lipid, as well as for its ability to reduce the required glucose load and associated VCO2. However, the hormonal environment of burn injury suppresses lipolysis and limits the utilization of lipids for energy. For this reason, most authorities recommend that fat comprise no more than about 30% of non-protein calories, or about 1 g/kg/day of intravenous lipids in TPN.14 Most of the products cited in Table 29.2 meet this target.
It may be desirable to give even less fat. In an animal model, adverse effects on immune function occurred when diets contained more than 15% lipids.49 Some clinical research supports this finding,50 leading some authorities to recommend very low-fat diets in burn patients. However, popular ‘stress’ enteral formulas typically contain 25–40% of calories as fat, and some ‘specialty’ formulas – for renal failure, respiratory failure, or diabetes – contain even more fat, which limits their usefulness in burn patients. Patients given TPN may be better off if fat is withheld entirely for short periods of time (as little as once weekly),1,51 though this may result in aggravated glucose intolerance.
The composition of administered fat may be even more important than the quantity. Most common sources contain mostly omega-6 fatty acids (FFAs) such as linoleic acid, which are metabolized through synthesis of arachidonic acid, a precursor of proinflammatory cytokines such as prostaglandin E2. Lipids such as fish oil containing a high proportion of omega-3 FFAs are metabolized without elaborating proinflammatory compounds. Diets high in omega-3 FFAs have been associated with improved immune response and possibly improved outcomes,52 and may reduce problems with hyperglycemia.53 Most experience with omega-3 FFAs has been obtained with ‘immune-enhancing diets,’ of which they are a major component (see below). Both the optimal composition and the optimal dose of fat in nutritional support remain topics of substantial controversy.
Protein
The hormonal environment of burn injury greatly increases proteolysis. Provision of carbohydrate and fat calories is only partially successful in reducing protein catabolism; some loss of lean body mass is obligatory.54 Increased protein must be supplied both to satisfy ongoing demands and to provide substrate for wound healing, immunity, and other functions. When calories are limited, protein will be used as an energy source rather than to replenish lost protein stores. The converse, however, is not true: providing calories in excess of need will not lead to increased protein retention or synthesis – it will just lead to overfeeding, with its associated risks. Protein intake above required needs (as in extremely high-protein/low-carbohydrate diets) also results in protein’s use as a fuel source through gluconeogenic pathways.
Protein catabolism in burn patients can exceed 150 g/day, or almost a half-pound of skeletal muscle. Although feeding supranormal amounts of protein may not reduce breakdown of endogenous protein stores,55 it facilitates protein synthesis and reduces negative nitrogen balance. In burned children, a diet including increased protein (23% of total calories) was associated with significantly better immune function, less bacteremia, and increased survival56 than in a group who received 17% protein diets. As burn size increases, progressively more protein is required for positive nitrogen balance.57,58
Current recommendations call for 1.5–2.0 g/kg/day of protein for adult burn patients, and up to 3.0 g/kg/day in children.59,60 With provision of sufficient non-protein calories, this should result in a calorie:nitrogen ratio of 100 : 1 or less, typical of the ‘stress’ formulas listed in Table 29.2. Measurement of nitrogen balance and visceral protein markers may be helpful in assessing the adequacy of nutritional support (see below).
Glutamine
Several amino acids have unique roles in energy delivery following injury. Alanine (ALA) and glutamine (GLU) are important transport amino acids, elaborated in large quantities from skeletal muscle to supply energy to the liver and healing wounds.54 GLU also serves as a primary fuel for both enterocytes and lymphocytes and is thus important in maintaining small bowel integrity, preserving gut-associated immune function and reducing intestinal permeability following injury.3,61,62 Glutamine is also a precursor of glutathione, an important antioxidant, and improves elaboration of heat shock proteins which provide cellular protection following stress and trauma.61,63–65
GLU is quickly depleted from both serum and muscle following burn injury, leading to the suggestion that GLU be considered a ‘conditionally essential’ amino acid in burns.54,66 GLU is almost entirely absent from parenteral nutrition owing to its instability in solution, which may explain some of the inferiority of TPN. GLU supplementation has been associated with some benefits when used in neutropenic cancer and other patients maintained on TPN.67,68
In clinical trials GLU supplementation has also produced some improved outcomes in patients with cancer, AIDS, surgery, trauma, or burns.3,69 However, these benefits were not consistently seen when comparing post-operative and ICU patients, provision of ‘low-dose’ (<0.20 g/kg/day) vs ‘high dose’ (>0.20 g/kg/day) GLU, or parenteral vs enteral administration.63 Some of the best data supporting GLU supplementation come from burn patients, in whom provision of 25 g GLU/kg/day or more, given parenterally70 or enterally,71,72 was associated with reduced infections, improved visceral protein levels, and reduced mortality and length of hospitalization. These effects of GLU may be relatively specific to burn injuries.73
Supplementation of enteral nutrition with GLU for burn patients is not routinely practiced, although this is becoming more common.74 The amounts of GLU given in clinical trials are so large (0.25–0.50 g/kg/day) that this could interfere with the delivery of other amino acids, so it might be preferable to give GLU in addition to the usual quantity of protein required by burn patients.3 Obviously, many questions persist regarding the clinical utility of GLU supplementation in burn care. Further clinical trials with supplementation of this amino acid are ongoing.
Arginine
Arginine (ARG) is also important in post-burn metabolism. ARG is synthesized from GLU and stimulates T lymphocytes, enhances natural killer cell function, and stimulates synthesis of nitric oxide (NO), which is important in stimulating inflammation and resistance to infection.75–77 ARG supplementation of enteral diets has been associated with improved immune responsiveness and wound healing,78 and improved survival in burned guinea pigs.79
Branched-chain amino acids
The branched-chain amino acids (BCAAs) leucine, isoleucine, and valine have been postulated to spare muscle catabolism by stimulating protein synthesis and serving as energy substrates. In clinical trials in trauma and ICU patients, BCAA-enriched nutrition was associated with improved nitrogen balance but had no effect on survival.80 In both animal and clinical studies in burn injury, BCAA-enriched feeding did not produce improvements in outcome, protein synthesis, or immune function,81 and they are therefore not recommended for use.
‘Immune-enhancing’ diets
Even the earliest efforts to nourish burn patients sought to create an ‘ideal’ nutritional mixture by supplementing diets with eggs or milk.26 Modern efforts have focused on combining components that appear to improve immune function and wound healing. In a landmark study, Gottschlich et al.82 found that a group of severely burned children given a custom-made tube feeding containing omega-3 FFAs, ARG, histidine, and vitamins A and C had significantly fewer wound infections, shorter hospital stays, and a trend toward improved survival compared to two control groups fed commercially available tube feedings. This experience quickly led to commercial production of similar multi-ingredient ‘immune-enhancing diets’ (IEDs).
Subsequent studies of IEDs in a variety of clinical settings have produced contrary and controversial results. Some have demonstrated improvements in immunity and wound healing, and reductions in infections, multiple organ failure, or hospital stay.83,84 No effect on mortality has been documented, however, and others have not been uniformly favorable. These diets may have very different consequences in different patient groups, with improved outcomes following trauma or elective surgery85,86 but deleterious or no effects in patients with sepsis or pneumonia.1,44,83 Much of the blame for these inconsistent results has been attributed to ARG, the exact dosing of which may influence its effects greatly, as too much could stimulate excessive NO production, exaggerate inflammation, and increase pulmonary dysfunction.87
Little information has been obtained on the use of available IEDs in burn patients. One small study compared a highly publicized IED (Impact™) with another commercially available, high-protein stress solution (Replete™),88 and found no differences in major outcome variables. Both solutions contained significant amounts of omega-3 FFAs and significant fat calories, though they differed in their contents of vitamin A, arginine, and RNA. It should be noted that no benefit to IEDs have been found in reducing the incidence of pneumonia,83 which is a frequent and serious problem for burn patients, particularly those suffering inhalation injury. Finally, it should be remembered that the high volume of feedings required by burn patients may mean that a satisfactory dose of some immune-enhancing nutrients is delivered even with the use of conventional diets.
These experiences illustrate that our understanding of nutritional physiology is still limited and may have been hindered by the rush to create commercially viable ‘cocktails’ for clinical use before understanding the real actions (and interactions) of their components and their optimal doses. At present the use of IEDs, particularly ARG, is controversial, being specifically recommended for burn patients in one authoritative practice guideline,4 and specifically not recommended in another.5 This issue must await clarification from future well-designed and large-scale clinical trials. The emerging field of ‘pharmaconutrition,’ which stresses more specific and restricted use of nutrients, may benefit this effort.48 It is safe to say that efforts to provide adequate amounts of calories and high-quality protein remain the mainstay of successful burn nutrition, rather than an emphasis on a specific formula.
Micronutrients: vitamins and trace elements
In addition to major nutrients, the metabolism of many so-called ‘micronutrients’ – vitamins and trace elements, which are important in wound healing and immunity – is also affected by burn injury.89 These compounds have not been evaluated extensively in clinical studies, although depressed serum levels of some compounds have been documented following burns. A complete list of micronutrients and their functions is provided in another chapter; excellent reviews are also available.89,90 Limited data suggest that supplementation of some substances, including vitamins A91,92 and C90) may be beneficial. Additional recent research has focused on disorders of bone and vitamin D metabolism,93 zinc,94 and selenium.95
A recent survey found that many burn centers provide some supplementation of trace elements, though perceived indications and doses varied widely.96 Current reviews recommend supplementing at least the micronutrients listed above, and perhaps others.15 However, many commercial tube feedings contain substantial quantities of these micronutrients, which can approximate the recommendations listed by Mayes et al.92 The addition of a daily multivitamin tablet or liquid multivitamins to tube feedings or TPN will provide far more than DRI recommendations for most micronutrients at minimal cost and risk. It is unclear whether additional supplementation is of any value.
Formulas for enteral nutrition
Successful nutrition has been provided to burn patients with very simple concoctions of milk, eggs, and other nutrients.26 However, commercially prepared enteral formulas offer several advantages. They are nutritionally complete, and can be infused through small feeding tubes with minimal clogging. Most are reasonably inexpensive, though some specialized formulas can be quite costly.
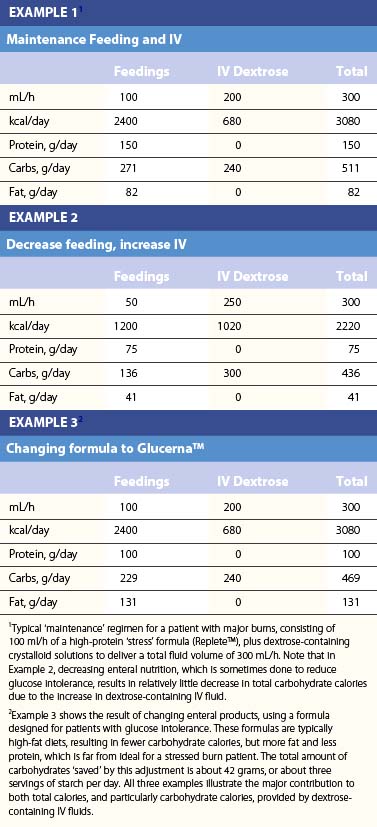
Table 29.2 lists the nutrient composition of a number of popular enteral formulas. A bewildering array of products are available, including fiber-containing diets, elemental diets, inexpensive supplements, and costly specialized diets for patients with renal failure, hepatic failure, glucose intolerance, etc. Many of these products do not meet the macronutrient needs of burn patients and often limit protein while providing excess fat and carbohydrate. As an example, Table 29.3 shows the effects of either changing the volume of enteral nutrition and intravenous fluids (a major source of calories for some patients), or switching to one of the ‘diabetic’ formulas available. Clearly, the choice of formula can greatly influence both the success of nutrition and the type and magnitude of complications encountered.
Formulas for TPN
The exact composition of TPN solutions can be tailored to individual patient needs. ‘Standard’ TPN solution (70% dextrose, 15% amino acids, and 20% lipid emulsion) may simplify ordering but often require customizing to meet the needs of burn patients, who are likely to require increased amounts of protein and possibly fewer lipid calories. Electrolytes, vitamins, and minerals can be custom ordered, or standard ‘packages’ can be used. Medications such as insulin and H2 blockers can be added as well. Box 29.1 is an example of a protocol for ordering and administering TPN used for a typical burn patient in our unit.
Box 29.1 Regimen for instituting total parenteral nutrition, adapted from ‘Adult parenteral nutrition orders’, University of Utah
Step One: Calculated Required Energy and Protein Needs
Example: 25 year-old man, 80 kg in weight. Body surface area = 2.2m2.
Step Two: Order TPN Solution
1 Carbohydrates: Carbohydrates are supplied as 70% dextrose (D70), which contains 2.4 kcal/mL. To give 75% of non-protein calories as dextrose, calculate:
2 Fat: The remainder of non-protein calories will be given as lipid emulsion. This is commonly available as 10% (1.0 kcal/mL) or 20% (2.0 kcal/mL) solution. To give 25% of non-protein calories as lipid, calculate:
3 Protein: Protein is supplied as crystalline amino acid solutions at a concentration of 10% (0.1 g/mL), or 15% (0.15 g/mL). To give 160 g of protein, calculate:
4 Total volume = 900 mL D70 + 360 mL lipids + 1067 mL amino acids = 2327 mL.
5 Add electrolytes: These can be ordered as customized additions in any quantity, but a standard electrolyte ‘package’ contains (per liter of TPN):
6 Add vitamins: A standard vitamin ‘package’ contains approximately 100% of recommended dietary allowances of vitamins A, C, D, E, and B12, pyridoxine, thiamine, riboflavin, niacin, pantothenic acid, folate, and biotin.
7 Add trace elements: A standard ‘package’ contains
8 Add customized components: These often include famotidine for stress ulcer prophylaxis, and insulin for glucose intolerance.
9 Add additional water, and calculate infusion rate: If patients require additional fluid, water can be added to the TPN solution as desired. If no additional water is required, then the goal rate for infusion is 2327 mL ÷ 24 h = 97 mL/h.
Step Four: Begin Monitoring on Nutritional Support
1 As infusion is initiated, measure blood glucose every 4 hours. If glucose is ≥ 120 mg/dL, begin administration of exogenous insulin by ‘sliding scale’, or continuous drip. Consider oral hypoglycemic agents.
2 After goal rate is achieved, measure blood glucose every 6 h.
Daily: Serum electrolytes, Blood urea nitrogen, creatinine.