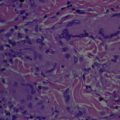
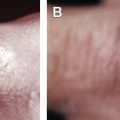
Skin and soft tissue infections caused by nontuberculous mycobacteria are increasing in incidence. The nontuberculous mycobacteria are environmental, acid-fast bacilli that cause cutaneous infections primarily after trauma, surgery and cosmetic procedures. Skin findings include abscesses, sporotrichoid nodules or ulcers, but also less distinctive signs. Important species include Mycobacterium marinum and the rapidly growing mycobacterium: M. fortuitum , M. abscessus and M. chelonae . Obtaining tissue for mycobacterial culture and histopathology aids diagnosis. Optimal therapy is not well-established, but is species-dependent and generally dictated by susceptibility studies. Management often includes use of multiple antibiotics for several months and potential use of adjunctive surgery.
Key points
- •
Skin and soft tissue infections caused by nontuberculous mycobacteria (NTM), especially the rapidly growing mycobacteria, appear to be increasing in incidence.
- •
Consider NTM as a cause of skin and soft tissue infection after trauma, surgery, or a cosmetic procedure, especially if the infection is not responding to typical antibiotic regimens.
- •
Skin signs can include abscesses, sporotrichoid nodules, or ulcers, but may not be distinctive, necessitating a high index of clinical suspicion.
- •
Obtain tissue cultures and susceptibility studies specifically for mycobacteria.
- •
Management is via prolonged antibiotic treatment that is species specific, generally based on antimicrobial susceptibility studies and may include surgical intervention.
Introduction
Definition and Classification
Mycobacteria species other than those of the Mycobacterium tuberculosis complex or Mycobacterium leprae are known as nontuberculous mycobacteria (NTM), environmental mycobacteria, or atypical mycobacteria. NTM are a diverse group of ubiquitous, environmental, acid-fast organisms that can produce a wide range of diseases, including infections of the skin and soft tissues. More than 170 species of NTM have been identified, most of which have been incriminated in skin and soft tissue infections (SSTI). Traditionally, NTM have been classified into Runyon groups based on colony morphology, growth rate, and pigmentation. As technology moves forward, this classification system has become less useful and identification is now made using rapid molecular diagnostic systems. Nonetheless, growth rates continue to provide practical means for grouping species of NTM. On this basis, NTM can be categorized into rapidly growing mycobacteria (RGM) and slowly growing mycobacteria (SGM).
RGM include species that produce mature growth on media plated within 7 days. These are subdivided into 5 groups based on pigmentation and genetic similarity: Mycobacterium fortuitum, Mycobacterium chelonae/abscessus, Mycobacterium mucogenicum, Mycobacterium smegmatis , and early pigmenting RGM. SGM include species of mycobacteria that require more than 7 days to reach mature growth. Examples of SGM are Mycobacterium marinum, Mycobacterium ulcerans, Mycobacterium kansasii, Mycobacterium haemophilum, and Mycobacterium scrofulaceum. Some species require nutritional supplementation of routine mycobacteria media, grow best at lower/higher temperatures or require prolonged incubation.
Most NTM species are easily isolated from the environment, including water (both natural and municipal systems), soil, plants, animals, and birds. Exceptions to this include M haemophilum and M ulcerans , which are rarely isolated. Tap water is considered the major reservoir for NTM pathogens in humans and as such is of increasing public health concern. Species typically recovered from tap water include Mycobacterium gordonae, M kansasii, Mycobacterium xenopi, Mycobacterium simiae, Mycobacterium avium complex (MAC), and the RGM. NTM develop and are protected within biofilms, the filmy layer between the solid and liquid interface, in municipal water systems. Carson and colleagues showed that 83%of the incoming city water in hemodialysis centers throughout the United States contained NTM. The presence of mycobacteria in up to 90% of samples taken from piped water systems has been described. Furthermore, biofilms may make the mycobacteria resistant to common disinfectants. NTM are difficult to eradicate with common decontamination techniques and are relatively resistant to standard disinfectants such as chlorine, glutaraldehyde, gigasept, and virkon. They can grow in hot and cold water systems. In some cases, temperatures of up to 70°C are required to inhibit the organism. Importantly, no evidence of person-to-person spread has been reported with NTM.
Clinical Syndromes
Four clinical syndromes account for most infections with NTM: pulmonary disease, lymphadenitis, disseminated disease, and SSTIs.
Pulmonary disease
The most common form of localized NTM infection is chronic pulmonary disease in human immunodeficiency virus (HIV)-negative hosts. Signs and symptoms of NTM lung disease are often nonspecific, making this a challenging diagnosis that requires extensive laboratory and imaging workup. MAC followed by M kansasii , and M abscessus are the most common pathogens in the United States.
Lymphadenitis
Localized cervical lymphadenitis is the most common NTM disease in children and is typically caused by MAC and M scrofulaceum . It occurs in children between 1 and 5 years of age. The cervicofacial nodes, particularly the submandibular nodes, are most frequently involved. These can enlarge rapidly with the formation of fistulas to the skin, and prolonged drainage may occur. As with all other NTM infections, definitive diagnosis of lymphadenitis is made by recovery of the etiologic organism from cultures.
Disseminated Disease
Disseminated NTM infections occur almost exclusively in immunocompromised patients.
Disseminated disease in patients with human immunodeficiency virus
Although M tuberculosis continues to be the most prevalent mycobacterial disease in HIV-AIDS, disseminated NTM is well documented and is associated with increased mortality in this patient population. The most commonly implicated NTM is MAC and although the incidence has decreased significantly with the introduction of highly active antiretroviral therapy, it remains an important complication of AIDS. M kansasii, Mycobacterium genavense, M scrofulaceum, M xenopi, M fortuitum, and M gordonae are among many other NTM responsible for disseminated disease in patients with HIV. Symptoms are not specific and in most cases resemble those seen in disseminated tuberculosis. These include intermittent or persistent fever, night sweats, weight loss, fatigue, malaise, and anorexia.
Disseminated disease in the severely immunocompromised
Disseminated disease in patients without HIV is rare and seen in the setting of significant immunosuppression (eg, transplant recipients, chronic corticosteroid use, leukemia). Systemic dissemination of a primary cutaneous NTM can occur. In most cases, disseminated disease presents with disseminated cutaneous lesions. The RGM species M chelonae is the most commonly isolated organism, presenting with multiple, red, draining, subcutaneous nodules or abscesses. M kansasii, M haemophilum, M fortuitum, M abscessus, and others have also been reported.
Skin and soft tissue infections
The increasing reports of SSTI NTM infections in recent years have attracted significant attention in the medical community. Initially thought to reflect the increased immunosuppressed population, numerous reports document infection in healthy individuals. The exact incidence of SSTI NTM infections is yet to be determined. The largest population-based study on the incidence of NTM, from Olmsted County, Rochester, MN, showed an incidence of 2.0 per 100,000 person-years, and a nearly threefold increase in the incidence of cutaneous NTM infections over a 30-year period. RGM were more predominant in the last decade of the study. This is supported by multiple publications that show an upward trend in all forms of NTM infections. In recent studies, NTM account for 15% of total isolates of acid-fast bacilli (AFB) with the remaining 85% M tuberculosis . Population-based studies in Spain showed that NTM infections represented 0.64% to 2.29% of all mycobacterial infections.
SSTIs caused by NTM include 2 distinctive species-specific clinical disorders: “fish-tank” granuloma and Buruli ulcer (BU), caused by M marinum and M ulcerans , respectively. However, most SSTIs caused by NTM are nonspecific in their clinical presentations and may present with abscesses, cellulitis, nodules, sporotrichoid nodules, ulcers, panniculitis, draining sinus tracts, folliculitis, papules, and plaques. The polymorphous manifestations of cutaneous NTM make the diagnosis difficult and a high index of suspicion in the appropriate clinical setting ( Table 1 ) is necessary to make a prompt diagnosis. NTM infections should be considered in all patients with “therapy resistant” SSTIs. Cutaneous NTM infections typically develop after traumatic injury, surgery, or cosmetic procedures. As reviewed previously, they also can occur secondarily as a consequence of a disseminated mycobacterial disease, especially among immunosuppressed patients. Although RGM have a weaker pathogenicity than SGM, they also can cause disseminated diseases in immunocompromised hosts. The etiopathogenesis, clinical presentation, evaluation, and management ( Table 2 ) of the NTM commonly responsible for SSTI are discussed in detail herein.
| Type of Mycobacteria | Clinical Setting |
|---|---|
| Slow-growing mycobacteria | |
| Mycobacterium marinum |
|
| Mycobacterium ulcerans |
|
| Mycobacterium kansasii |
|
| Mycobacterium haemophilum |
|
| Rapid-growing mycobacteria | |
| Mycobacterium fortuitum Mycobacterium abscessus Mycobacterium chelonae |
|
| Type of Mycobacteria | Treatment | Level of Evidence |
|---|---|---|
| Slow-growing mycobacteria | ||
| Mycobacterium marinum |
| D, E |
| Mycobacterium ulcerans |
| E |
| Mycobacterium kansasii |
| C, E |
| Mycobacterium haemophilum |
| D, E |
| Rapid-growing mycobacteria | ||
| Mycobacterium fortuitum Mycobacterium abscessus Mycobacterium chelonae |
| E |
Introduction
Definition and Classification
Mycobacteria species other than those of the Mycobacterium tuberculosis complex or Mycobacterium leprae are known as nontuberculous mycobacteria (NTM), environmental mycobacteria, or atypical mycobacteria. NTM are a diverse group of ubiquitous, environmental, acid-fast organisms that can produce a wide range of diseases, including infections of the skin and soft tissues. More than 170 species of NTM have been identified, most of which have been incriminated in skin and soft tissue infections (SSTI). Traditionally, NTM have been classified into Runyon groups based on colony morphology, growth rate, and pigmentation. As technology moves forward, this classification system has become less useful and identification is now made using rapid molecular diagnostic systems. Nonetheless, growth rates continue to provide practical means for grouping species of NTM. On this basis, NTM can be categorized into rapidly growing mycobacteria (RGM) and slowly growing mycobacteria (SGM).
RGM include species that produce mature growth on media plated within 7 days. These are subdivided into 5 groups based on pigmentation and genetic similarity: Mycobacterium fortuitum, Mycobacterium chelonae/abscessus, Mycobacterium mucogenicum, Mycobacterium smegmatis , and early pigmenting RGM. SGM include species of mycobacteria that require more than 7 days to reach mature growth. Examples of SGM are Mycobacterium marinum, Mycobacterium ulcerans, Mycobacterium kansasii, Mycobacterium haemophilum, and Mycobacterium scrofulaceum. Some species require nutritional supplementation of routine mycobacteria media, grow best at lower/higher temperatures or require prolonged incubation.
Most NTM species are easily isolated from the environment, including water (both natural and municipal systems), soil, plants, animals, and birds. Exceptions to this include M haemophilum and M ulcerans , which are rarely isolated. Tap water is considered the major reservoir for NTM pathogens in humans and as such is of increasing public health concern. Species typically recovered from tap water include Mycobacterium gordonae, M kansasii, Mycobacterium xenopi, Mycobacterium simiae, Mycobacterium avium complex (MAC), and the RGM. NTM develop and are protected within biofilms, the filmy layer between the solid and liquid interface, in municipal water systems. Carson and colleagues showed that 83%of the incoming city water in hemodialysis centers throughout the United States contained NTM. The presence of mycobacteria in up to 90% of samples taken from piped water systems has been described. Furthermore, biofilms may make the mycobacteria resistant to common disinfectants. NTM are difficult to eradicate with common decontamination techniques and are relatively resistant to standard disinfectants such as chlorine, glutaraldehyde, gigasept, and virkon. They can grow in hot and cold water systems. In some cases, temperatures of up to 70°C are required to inhibit the organism. Importantly, no evidence of person-to-person spread has been reported with NTM.
Clinical Syndromes
Four clinical syndromes account for most infections with NTM: pulmonary disease, lymphadenitis, disseminated disease, and SSTIs.
Pulmonary disease
The most common form of localized NTM infection is chronic pulmonary disease in human immunodeficiency virus (HIV)-negative hosts. Signs and symptoms of NTM lung disease are often nonspecific, making this a challenging diagnosis that requires extensive laboratory and imaging workup. MAC followed by M kansasii , and M abscessus are the most common pathogens in the United States.
Lymphadenitis
Localized cervical lymphadenitis is the most common NTM disease in children and is typically caused by MAC and M scrofulaceum . It occurs in children between 1 and 5 years of age. The cervicofacial nodes, particularly the submandibular nodes, are most frequently involved. These can enlarge rapidly with the formation of fistulas to the skin, and prolonged drainage may occur. As with all other NTM infections, definitive diagnosis of lymphadenitis is made by recovery of the etiologic organism from cultures.
Disseminated Disease
Disseminated NTM infections occur almost exclusively in immunocompromised patients.
Disseminated disease in patients with human immunodeficiency virus
Although M tuberculosis continues to be the most prevalent mycobacterial disease in HIV-AIDS, disseminated NTM is well documented and is associated with increased mortality in this patient population. The most commonly implicated NTM is MAC and although the incidence has decreased significantly with the introduction of highly active antiretroviral therapy, it remains an important complication of AIDS. M kansasii, Mycobacterium genavense, M scrofulaceum, M xenopi, M fortuitum, and M gordonae are among many other NTM responsible for disseminated disease in patients with HIV. Symptoms are not specific and in most cases resemble those seen in disseminated tuberculosis. These include intermittent or persistent fever, night sweats, weight loss, fatigue, malaise, and anorexia.
Disseminated disease in the severely immunocompromised
Disseminated disease in patients without HIV is rare and seen in the setting of significant immunosuppression (eg, transplant recipients, chronic corticosteroid use, leukemia). Systemic dissemination of a primary cutaneous NTM can occur. In most cases, disseminated disease presents with disseminated cutaneous lesions. The RGM species M chelonae is the most commonly isolated organism, presenting with multiple, red, draining, subcutaneous nodules or abscesses. M kansasii, M haemophilum, M fortuitum, M abscessus, and others have also been reported.
Skin and soft tissue infections
The increasing reports of SSTI NTM infections in recent years have attracted significant attention in the medical community. Initially thought to reflect the increased immunosuppressed population, numerous reports document infection in healthy individuals. The exact incidence of SSTI NTM infections is yet to be determined. The largest population-based study on the incidence of NTM, from Olmsted County, Rochester, MN, showed an incidence of 2.0 per 100,000 person-years, and a nearly threefold increase in the incidence of cutaneous NTM infections over a 30-year period. RGM were more predominant in the last decade of the study. This is supported by multiple publications that show an upward trend in all forms of NTM infections. In recent studies, NTM account for 15% of total isolates of acid-fast bacilli (AFB) with the remaining 85% M tuberculosis . Population-based studies in Spain showed that NTM infections represented 0.64% to 2.29% of all mycobacterial infections.
SSTIs caused by NTM include 2 distinctive species-specific clinical disorders: “fish-tank” granuloma and Buruli ulcer (BU), caused by M marinum and M ulcerans , respectively. However, most SSTIs caused by NTM are nonspecific in their clinical presentations and may present with abscesses, cellulitis, nodules, sporotrichoid nodules, ulcers, panniculitis, draining sinus tracts, folliculitis, papules, and plaques. The polymorphous manifestations of cutaneous NTM make the diagnosis difficult and a high index of suspicion in the appropriate clinical setting ( Table 1 ) is necessary to make a prompt diagnosis. NTM infections should be considered in all patients with “therapy resistant” SSTIs. Cutaneous NTM infections typically develop after traumatic injury, surgery, or cosmetic procedures. As reviewed previously, they also can occur secondarily as a consequence of a disseminated mycobacterial disease, especially among immunosuppressed patients. Although RGM have a weaker pathogenicity than SGM, they also can cause disseminated diseases in immunocompromised hosts. The etiopathogenesis, clinical presentation, evaluation, and management ( Table 2 ) of the NTM commonly responsible for SSTI are discussed in detail herein.
| Type of Mycobacteria | Clinical Setting |
|---|---|
| Slow-growing mycobacteria | |
| Mycobacterium marinum |
|
| Mycobacterium ulcerans |
|
| Mycobacterium kansasii |
|
| Mycobacterium haemophilum |
|
| Rapid-growing mycobacteria | |
| Mycobacterium fortuitum Mycobacterium abscessus Mycobacterium chelonae |
|
| Type of Mycobacteria | Treatment | Level of Evidence |
|---|---|---|
| Slow-growing mycobacteria | ||
| Mycobacterium marinum |
| D, E |
| Mycobacterium ulcerans |
| E |
| Mycobacterium kansasii |
| C, E |
| Mycobacterium haemophilum |
| D, E |
| Rapid-growing mycobacteria | ||
| Mycobacterium fortuitum Mycobacterium abscessus Mycobacterium chelonae |
| E |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree