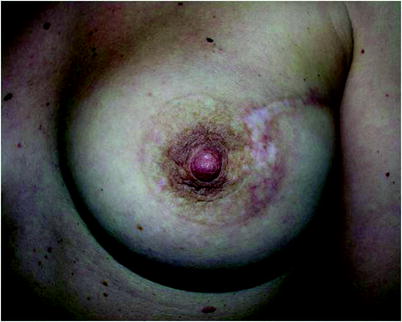
Fig. 25.1
Skin incision
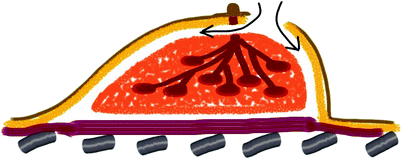
Fig. 25.2
Glandular dissection
All patients with N0 cancer undergo sentinel lymph node biopsy, and if the findings are positive, axillary dissection is conducted. Node-positive patients undergo an axillary node dissection.
25.2.2 Pathological Intraoperative Examination
The retroareolar specimen is cored out from underneath the nipple (Fig. 25.3). The tumour site of the retroareolar specimen is inked and sent to the pathologist for frozen section examination. The minimum of 5-mm thickness is required behind the areola to reduce the risk of NAC necrosis [12]. The confirmation of negative findings is required to complete preservation of the NAC and conduct the ELIOT procedure.
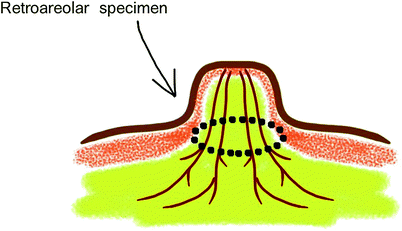

Fig. 25.3
Retroareolar specimen sampling
Artefacts caused by freezing may reduce the reliability of analysis of frozen sections of epithelial cells of the ducts. Invasive carcinoma or ductal carcinoma in situ and subtle cellular or architectural abnormalities might be more difficult to assess in frozen sections than in definitive sections. Lohsiriwat et al. [14] showed that the frozen section of subnipple tissue has specificity of 96.6, sensitivity of 88.2 and accuracy of 93.5 %, whereas Benediktsson and Perbeck [15] found specificity of 98.5 %. Some authors have proposed a histological retroareolar evaluation before the mastectomy by a surgical biopsy or by a mammotome in place of the traditional frozen section [16–18]. In our experience, we observed 8.2 % false-negative results [11].
25.2.3 ELIOT Technique
At the European Institute of Oncology, one electron beam shot is delivered by a linear accelerator in all patients in whom the NAC was preserved. Our protocol includes ELIOT on the NAC according to the linear-quadratic model (with an α/β ratio of 4 for breast cancer) equivalent to a fractionated dose ranging from 40 to 45 Gy. The clinical target volume, fully encompassed by the collimator, is the diameter of the areola plus a 1-cm margin around it. The entire target is included in the 90 % isodose [11, 19–21]. The lead disk and aluminium disk are positioned beneath the NAC and on the surface of the pectoralis major muscle to prevent muscular and chest wall irradiation before delivering ELIOT. The radiologist and physical technician provide ELIOT in the operating theatre. However, irradiation of the NAC is postponed or cancelled if the blood supply after the subcutaneous mastectomy is critical (Fig. 25.4).
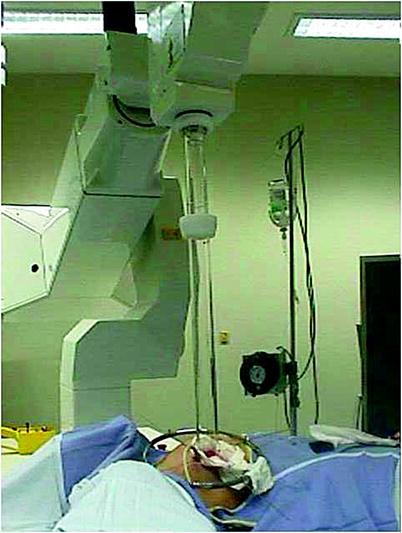

Fig. 25.4
Intraoperative radiotherapy setting
25.2.4 Reconstruction Technique
The plastic surgeon is called upon immediately to reconstruct the breast using one of the techniques adapted to the particular case: definitive implant, expander or musculocutaneous flaps. At the European Institute of Oncology, definitive one-step implant is the main procedure for total breast reconstruction if feasible and provides good results especially in the case of small or nonptotic breasts. (Figs. 25.5, 25.6) Surgeons aim for total submuscular coverage by creating a pocket under the pectoralis major muscle, pectoral fascia and serratus anterior muscle. To obtain a natural ptosis, the reconstruction can be performed with an autologous flap. (latissimus dorsi or transverse rectus abdominis myocutaneous flaps) The deepithelialized flap provides a live implant, avoiding the risk of contracture. The use of alloplastic material can also improve the natural shape and ptosis. The risk of reconstruction failure increases with the risk of skin necrosis and the size of the breast [22]. In our experience, deepithelialized autologous reconstruction can improve the blood supply of the NAC.
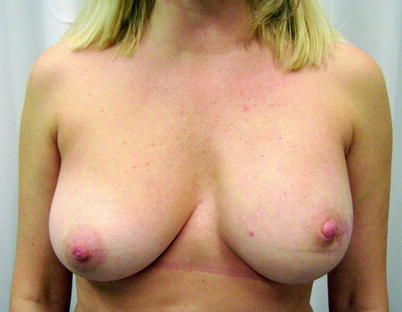
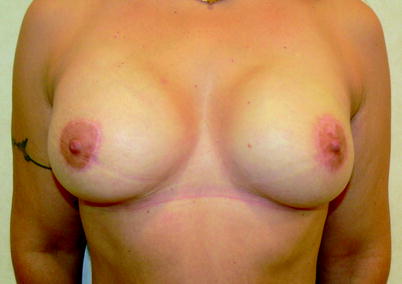

Fig. 25.5
Immediate breast reconstruction in a ptotic breast after nipple-sparing mastectomy

Fig. 25.6
Bilateral immediate breast reconstruction after nipple-sparing mastectomy
25.3 Complications
Infection and necrosis occur in 2–10 % of cases [11, 23, 24]. In our NSM series [11], total necrosis of the NAC was observed in 35 of the 1,001 NSM cases (3.5 %). Partial necrosis was observed in 55 cases (5.5 %). The NAC was removed in 50 cases (5.0 %).
Recently, we performed a prospective trial and measured the thickness of the mastectomy flap and NAC flap in 50 NSMs [12] (Fig. 25.7) We observed partial necrosis in 26.0 % of cases. Total necrosis was not observed. Superficial necrosis of the NAC and adjacent skin was observed. The necrosis involved the NAC and adjacent skin in 11 cases. In this recent series, the necrosis was also associated with young age (less than 45 years) and smoking. BMI, hypertension, diabetes mellitus and breast weight were not associated with necrosis. Moreover, the cut-off limit for the risk of necrosis was 5 mm.
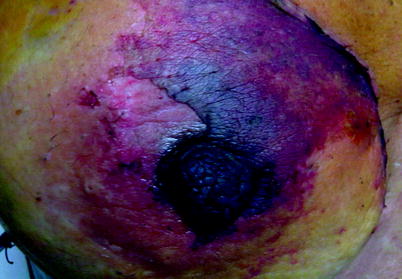

Fig. 25.7
NAC Necrosis
25.4 Oncological Outcome
Boneti et al. [25] did not find any statistical differences when comparing NSM and skin-spring mastectomy for locoregional recurrence rate (6 % vs. 5.0 % p = 0.89). Gerber et al. [26] did not observe any difference between the locoregional recurrence rate of modified mastectomy, skin-sparing mastectomy and NSM: 11.5 % versus 10.4 % versus 11.7 %, respectively, at 8.4 years. The comparison of the results between the NSM series is questionable because of the different selection criteria (invasive or in situ, risk-reducing mastectomy included in the series), the different techniques (one-stage or delayed NSM), the use of intraoperative radiotherapy and the difference in follow-up.
In a recent study of 934 consecutive NSM patients during 2002–2007, median follow-up was 50 months. In 772 invasive carcinoma patients, the rate of locoregional recurrence in the breast and in the NAC was 3.6, and 0.8 %, respectively. In the 162 patients with intraepithelial neoplasia, the rate of locoregional recurrence in the breast and in the NAC was 4.9, and 2.9 %, respectively. The significant risk factors for locoregional recurrence in the breast for invasive carcinoma were grade, overexpression/amplification of human epidermal growth factor receptor 2 (HER2)/neu and breast cancer molecular subtype luminal B. In the intraepithelial neoplasia group, the risk factors for locoregional recurrence in the breast and in the NAC were age (less than 45 years), absence of oestrogen receptors, grade, HER2/neu overexpression and high Ki-67 level. We conclude that the locoregional recurrence rate after NSM in our series was low but the biological features of disease and young age should be taken into account when considering indications for NSM in breast cancer patients [27].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree