Fig. 26.1
Partial cellular and molecular immunological mechanisms in lesional skin of AD. Factors surrounded by red square are targets of therapeutic approaches described in this manuscript. ‘Acute’ shows acute phase of AD and ‘Chronic’ shows chronic phase of AD. In acute phase of AD skin lesion, Th2-type lymphocytes should be dominated and produce Th2-type cytokines, such as IL-4, IL5 and IL-13. In chronic phase of AD skin lesion, mixed-type Th lymphocytes (Th1, Th17, Th22 dominated and also Th2) should be infiltrated. Solid arrows show positive regulation, and dotted arrows show negative regulation. AMPs antimicrobial peptides, TSLP thymic stromal lymphopoietin, Eo eosinophils, MC mast cells, LC: Langerhans cells, DC dendritic cells
26.2 Approach from a ‘Barrier Disorder’
26.2.1 Physical Barrier
As seen from the fact that one of the three pillars of AD treatment advocated by the Japanese Dermatological Association is ‘skin care’, skin barrier dysfunction has been widely recognised as an important element of the pathogenesis of AD, and various studies concerning the topic have been conducted [1, 2]. Since the relationship between filaggrin gene mutations and AD was reported in 2006, the examination of the pathology of AD from this aspect has gained further attention.
The following possible effects of filaggrin mutation on AD have been indicated. Because filaggrin is one of the important elements in skin barrier formation and when mutation is present, there is an increased possibility that at least the skin barrier function decreases, facilitating exogenous invasion and sustained intrusion of the allergen, which may contribute to the onset or exacerbation of AD.
This speculation is supported by data from research [3–6], and it appears that the actual conditions are reflected by these results; however, because many studies have reported that filaggrin gene mutations occur in ~30% of all AD cases and there are many individuals with filaggrin gene mutation who have normal traits, it may be safer to say that decreased filaggrin expression in the skin is one of the many factors that lead to the onset of AD.
However, the existence of a mutation in the filaggrin gene results in a certain degree of loss of skin barrier function in which the expression of filaggrin is reduced in the skin and as previously mentioned can be expected to be a possible risk factor for continued skin problem; it can be assumed that by screening filaggrin gene mutations, it will be possible to elucidate one of the multiple risk factors of AD.
26.2.2 Functional Barrier
As previously mentioned, the skin has both a so-called physical barrier, composed of filaggrin and other proteins, and a functional barrier that inhibits the infection or fixation of pathogenic microorganisms. Peptides that exhibit antibacterial or antiviral activities, which are collectively referred to as antimicrobial peptides (AMPs), are produced from epidermal keratinocytes and contribute to the primary biological defence of the skin surface [9–11]. Expression of LL-37 and human beta-defensin (hBD)-2 and hBD-3 that belong to AMPs has been reported to decrease in the local skin of patients with AD [12–14]. These findings suggest that it is one of the factors of bacterial fixation or infection in the skin surface of patients with AD that is commonly observed. It is often observed in the clinical practice that microbial infection of the skin exacerbates the symptoms of AD; even when infection is not present but fixation of pathogenic microorganisms has occurred, the possibility that the produced exotoxin may act as a superantigen and is involved in the exacerbation of the symptoms of AD by promoting the activation of nonspecific T cells has been indicated [15].
In this context, it has been reported that activated vitamin D3 increases the expression of AMPs in cultured keratinocytes [16]; in the case of some AMPs, vitamin D response elements in the promoter region of the gene [17, 18], elevated (recovered) AMP expression in the skin of patients with AD following oral vitamin D formulation administration and further amelioration of dermal symptoms have also been reported by other studies [19–22].
26.2.3 Others
There are in vitro data that reveal a reduction in the expression of proteins involved in the physical skin barrier (e.g. filaggrin) and AMPs (i.e. LL-37, hBD) involved in the functional skin barrier when the Th2 cytokine is present, even when there are no genetic mutations [12, 23, 24]. There are many patients with AD in which an abnormal increase in Th2-type immune response is thought to be a factor of the pathogenesis of the condition; based on these findings, it is possible that attempted treatments to correct the part of the abnormal enhancement of the Th2-type immune response in AD not only suppress the inflammatory reaction involved in this immune response but may also promote recovery of the skin barrier function.
26.3 Approach from Elevated Serum IgE Antibody Levels
As a result, the idea that increased Th2-type immune response, which may lead to increased production of IgE antibodies, is an onset or exacerbation factor of AD has long existed [25]. These findings form the basis for the examination of possible AD treatments targeting IgE antibodies.
In recent years, a subgroup with normal serum IgE antibody has been proposed [26], which is considered to be an effective method to categorise patients who are thought to have heterogeneous AD. However, such a group may not have treatment methods targeting IgE antibodies. Thus, by dividing AD into subgroups, it may be possible to discover specific treatments or management methods for each subgroup.
26.3.1 Anti-IgE Antibody (Omalizumab)
The fact that the subgroup with elevated serum IgE antibody levels comprise 80% of the total subgroup and the question of whether IgE antibodies are actively involved in the pathogenesis of AD remains, there are still concerns to discuss.
Regarding the findings that suggest the possibility that IgE antibodies are directly involved in the pathogenesis of AD, there are data that suggest that as a result of the presence of FcεRI-positive antigen-presenting cells in AD lesions, the antigen-presenting ability of the T cells of the antigen-presenting cells is dramatically enhanced, arising from intracellular signalling due to the presence of antigens and antigen-specific IgE antibodies [27]. These findings suggest the possibility of therapeutic effects of anti-IgE antibody (omalizumab) on AD. The treatment mechanism appears to be through trapping of the allergens by the immune complexes of the IgE antibody and omalizumab to prevent the activation of FcεRI-positive cells by the allergens [28].
However, several pilot and case studies have attempted to discover treatments for AD; of these, conflicting conclusions have been reported. It appears that therapeutic effects can be obtained through selection of target patients [29, 30], and the question whether clinical efficacy commensurate with the current cost can be obtained remains.
26.3.2 Intrinsic and Extrinsic ADs
Attempts were originally made to classify diseases as ‘intrinsic’ or ‘extrinsic’ starting with asthma [31]. This concept has similarly been utilised for AD, and so far, ‘allergic’ and ‘non-allergic’ types of AD have been reported. Also ‘intrinsic’ type of AD has been reported as ‘atopiform dermatitis’ [26, 32]. In all cases, it appears that antigen sensitisation by the IgE antibody does not occur, that is, there is a group in which nonspecific or antigen-specific IgE antibodies do not increase. It may be possible to classify this type as ‘intrinsic AD’ and all other types as ‘extrinsic AD’.
When such group classifications are made, various examinations are performed to examine the characteristics of each group, in addition to IgE antibody values. Reports on intrinsic AD to date have shown the characteristics of late-onset AD in women, as well as low severity, whereas extrinsic AD had a strong relationship with barrier dysfunction, which is characterised by early onset and has a comparatively high severity [33].
In recent years, classification based on types in which Th2-type immune reaction is dominant and types in which it is not dominant (Th1, 17, 22 mixed types) has been proposed, regardless of high or normal IgE antibody levels. Thus, these attempts aim to classify AD, a disease that is considered a loose collection of heterogeneous diseases, into subgroups through reconfiguration of uniform multiple groups as much as possible, and it is expected that the so-called tailor-made therapy can be established for each group and these attempts lead to ‘precision medicine’.
26.4 Approach from an Aim of ‘Reducing Inflammation’
Although various factors and mediated pathways exist in AD cases, clinical findings reveal somewhat uniform chronic inflammatory responses in the skin. Therefore, when considering treatments for AD, the necessity of the examination of inflammation reduction is clear. In fact, the most popular current pharmacotherapies for AD, such as topical steroids and ointments containing immunosuppressive agents, are a result of this approach.
26.4.1 Proactive Therapy Utilising Topical Agents
As stated above, topical steroids and topical agents containing immunosuppressive agents have a reliable suppressing effect on inflammation and are currently the primary (topical) pharmacotherapies used for AD. It has been reported that when these agents (standard remission induction therapy, which is so-called reactive therapy) are used, exacerbation of symptoms is significantly inhibited by continued proactive therapy through external use twice per week after remission induction [34, 35]. Although topical agents are already widely used, it may be possible to obtain better therapeutic effects through application of the preparations. As a result, the question of how long proactive therapy should continue arises; however, recent guidelines issued in Germany recommend the treatments to be administered initially for 3 months [36]. However, much accumulation of data is needed concerning these findings.
Ointments containing immunosuppressive agents are currently used for the treatment of various skin diseases, including AD; however, since the beginning, there have been concerns about the use of such ointments with regard to the risk of onset of lymphomas and local malignant tumours. Systematic reviews in 2015 reported that there is no evidence that actively suggests the onset of malignant tumours following such use [37, 38]. Although a satisfactory conclusion has been reached, further long-term observations may be necessary.
26.4.2 Others
Biological drugs that are already used in the clinical treatment of psoriasis in dermatology in Japan are predicted to be used in the treatment of AD. Although these are case and open-label studies, there are many reports available [39, 40]. TNF inhibitors that are already used in Japan include those in self-injection forms; such drugs may be applied to AD treatment in the future. As in the case of psoriasis, it is necessary to consider the possibility of safety problems in using such treatments (i.e. easy to infect or so-called paradoxical reactions).
26.5 Attempts to Apply Nucleic Acid Drugs to the Treatment of AD
Drugs with functional molecules that utilise nucleic acids with a variety of functions and are widely applied as pharmaceutical products are referred to as ‘nucleic acid drugs’ [41]. Currently, various attempts are being made in several fields. For example, antisense DNA, MicroRNA and decoy oligodeoxynucleotides (decoy ODNs) have been developed to inhibit gene expression and are used for various purposes [41]. Here, we explain the decoy ODNs and small interfering RNAs (siRNAs) that we also examined.
In the transcriptional regulatory region that controls the gene expression of DNA, a DNA sequence that binds with a specific transcription factor is artificially synthesised to obtain a double-stranded DNA fragment. This is made into decoy ODNs. When administered intracellularly, the transcriptional regulatory factors are competitively trapped by the decoy ODNs, thereby exerting inhibitory effect of gene expression (Fig. 26.2).
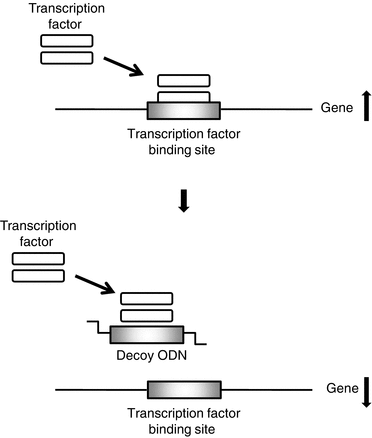

Fig. 26.2
Gene suppression mechanisms of decoy ODNs. A DNA sequence of binding site of transcription factor is artificially synthesised, and we used this as decoy ODNs. When administered intracellularly, the transcriptional factors are competitively trapped by the decoy ODNs, thereby exerting inhibitory effect of gene expression
RNA interference (RNAi) was originally a phenomenon discovered in nematodes, and a similar phenomenon was subsequently found to exist in various species. In short, when a double-stranded RNA is present, the phenomenon in which mRNA having a complementary base sequence is decomposed occurs [42]. Using this phenomenon to intracellularly introduce the artificially synthesised double-stranded RNA, the method of inhibiting the expression of a given gene has been established.
26.5.1 NF-kappaB Decoy ODNs
Morishita et al. reported that the results of gene suppression using decoy ODNs that target NF-kappaB, a transcription regulatory factor, revealed an amelioration in the conditions in an animal model of ischaemic heart disease [43]. Therapeutic effects of NF-kappaB decoy ODNs were also noted in animal models of AD [44], and a clinical trial for severe AD using an ointment containing NF-kappaB decoy ODNs has been conducted.
26.5.2 Attempts at Utilising Nucleic Acid Drugs That Target the Signal Transducer and Activator of Transcription (STAT) 6 for the Treatment of AD
AD is considered an intractable condition with various causes in each patient; however, there appear to be several candidate background factors with shared mechanisms. Filaggrin gene mutations, which have attracted much attention in recent years, may have at least some relationship with epidermal barrier dysfunction [45]. Thus, it can be surmised that this is one of the factors contributing to the treatment resistance of AD.
However, the overproduction of IgE antibodies is a phenomenon often seen in AD, and it has been previously considered by some that increased Th2-type immune response may be an exacerbating factor of AD onset. Although classifications can be made on the basis of intrinsic AD or non-type 2 immune response AD (normal IgE antibody levels) or extrinsic AD or type 2 immune response AD (elevated IgE antibody levels) [33], the dominance of Th2-type immunity has also been observed in the locally inflamed skin in intrinsic AD [46], regardless of IgE antibody status; it can be considered that increased Th2-type immune response is involved to a certain degree in all patients with AD.
We have previously focused on the pathogenesis of AD, which is based on this increase in Th2-type immune response. In 2000, Yokozeki et al. reported the intracellular signal transduction of IL-4 and IL-13 cytokines; by focusing on STAT6, which plays an important role in Th2-type immune response as a transcriptional regulatory factor, an interesting result that a prominent inhibition of hapten-induced contact hypersensitivity in knockout mice was noted [47]. These results suggest that Th2-type immune response plays as an active positive factor of induction of skin allergy inflammatory reaction. It has been subsequently confirmed that the STAT6-mediated response pathway is also actively involved in the pathogenesis in a mouse model of various skin allergic inflammatory reactions [48, 49]; by inhibiting this pathway, skin allergic inflammatory reactions may be treated. Specifically, a decoy nucleic acid (STAT6 decoy ODNs) was used to inhibit the function of the target, STAT6. We examined the effects of STAT6 decoy ODNs starting from local injections to finally in topical preparations; however, we concluded that allergic skin inflammatory responses in a mouse model were clearly suppressed by the administration of STAT6 decoy ODNs [48, 49].
It has also been reported that by simultaneously stimulating human epidermal keratinocytes, fibroblasts or vascular endothelial cells with IL-4, eotaxin or enhanced production of cell adhesion factor is observed [50, 51]. Furthermore, there is an accumulation of data that suggests that Th2-type immune response is actively involved in the increase of the inflammatory reactions in the local skin in mice and humans.
On the basis of these study results, a pilot study to examine the therapeutic effects of the inhibition of Th2-type immune response on skin conditions in actual patients with AD was conducted. Patients with moderate to severe adult AD with facial or trunk erythema were selected as subjects; however, therapeutic effects were observed in all subjects following the use of ointments containing STAT6 decoy ODNs (Fig. 26.3) [52].
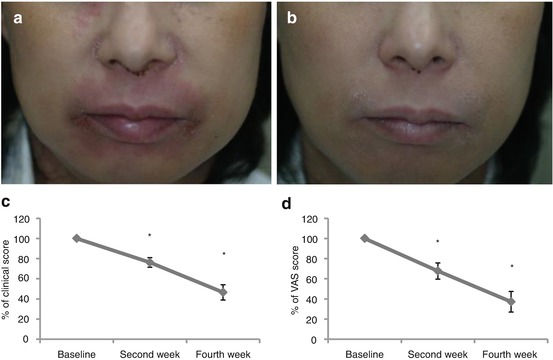

Fig. 26.3
Therapeutic trial of STAT6 decoy ODN ointment for AD. Clinical features of facial erythema in a representative case at baseline (a), at week 2 (b).The changes of clinical (c) and visual analogue score (VAS) scores for pruritus (d) for facial lesions. Data were expressed as average percentage of baseline scores ± SEM. *p < 0.05
Furthermore, it was reported that by utilising RNAi that targets STAT6 (in this case, short interfering RNA; STAT6 siRNA was created), mouse skin or nasal allergic inflammation reactions could be suppressed [53]. Thus, the possibility ~Th2-type immune response has been clearly indicated additionally, the utility of nucleic acid drugs using decoy ODNs and siRNA has been demonstrated as a method of treatment. Furthermore, excessive Th2-type immune response had recently been reported to inhibit the production of molecules responsible for skin barrier function [23, 54], and it is the authors’ opinion that our proposed concept of the treatment of AD by the inhibition of Th2-type immunity is a treatment method with higher specificity to pathology in comparison with conventional targeted therapies, such as topical steroids and topical agents containing immunosuppressive agents.
When developing nucleic acid drugs that target STAT6 for clinical use, the most critical problem was the high molecular weight of the nucleic acid drugs (10,000–20,000 Da). The skin has a considerably strict barrier function, and the size of substances that can relatively freely penetrate the skin from the outside is no >500 Da. [32]. In fact, in clinical studies using the previously mentioned ointment containing STAT6 decoy ODNs, efficacy was not observed in all cases; one reason for this appeared to be the possible permeability problems of nucleic acids in the skin. Moreover, if the problem of permeability can be solved in terms of therapeutic effects and economic aspects considering commercialisation of the product (if efficacy with a small amount of nucleic acid can be achieved, costs can be reduced), its utility may be greatly improved. Thus, the use of novel technologies with high efficacy that can introduce highly polymerised compounds, such as STAT6 decoy ODNs into the cells that comprise the skin through horny layer permeability, is currently being examined, and research concerning the development of nucleic acid drugs targeting STAT6 is progressing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree