NERVE INJURIES
I. PERIPHERAL NERVE ANATOMY (See Fig. 1-2)
A. Nerve fiber: The fundamental unit of the peripheral nervous system, which may be motor, sensory, or motor–sensory (mixed)
1. Motor (efferent) nerve fibers
a. Carries nerve signals away from the spinal cord to effector organs (e.g., muscle)
b. *The large, multipolar cell body of the motor neuron is located within the ventral horn of the spinal cord and is connected to a single, long axon that exits the spinal cord through the ventral root
c. Terminates on motor end plates within the innervated muscle
2. Sensory (afferent) nerve fibers
a. Carries nerve signals toward the spinal cord from highly specialized sensory end organs located in the skin and deeper tissues
b. The pseudounipolar cell body of the sensory neuron is located within the dorsal root ganglion and receives a single axonal process from the periphery (either from an encapsulated receptor or from a terminal sensory branch)
c. Terminates in the dorsal horn of the spinal cord, or may ascend to the brainstem
3. Autonomic nerve fibers
a. Control vasomotor and pilomotor function
b. Preganglionic autonomic nerve fibers (white rami) travel from the spinal cord to the ganglion and are cholinergic and myelinated
c. Postganglionic autonomic nerve fibers (gray rami) travel from the ganglion to effector organs, are unmyelinated, and are either cholinergic (parasympathetic division) or adrenergic (sympathetic division)
B. Nerve components
1. Nerve fiber proper
a. A filamentous extension (axon) of the motor or sensory neuron
b. Can be myelinated or unmyelinated
2. Schwann cells
a. Envelop single myelinated axons or multiple unmyelinated axons
b. Produce a myelin sheath to insulate axons and increase conduction velocity
c. Each Schwann cell covers approximately 1 mm of axonal length
d. Schwann cells are separated from one another by a small gap (node of Ranvier), which increases conduction velocity from 1 m/s to over 100 m/s by allowing action potentials to jump from node to node in a process known as saltatory conduction
3. Connective tissue
a. Comprises approximately 25% to 75% of the whole nerve cross-sectional area
b. Endoneurium
i. Surrounds individual axons within a fascicle
ii. Myelinated axons have diameters ranging from 3 to 20 μm
iii. Unmyelinated axons have diameters ranging from 0.2 to 1.5 μm
______________
*Denotes common in-service examination topics
c. Perineurium
i. Surrounds individual fascicles within the nerve
ii. Fascicles represent a group or arrangement of nerve fibers (usually less than 3 mm in diameter)
iii. The substance that is sutured together in fascicular repair
d. Epineurium
i. Outer epineurium surrounds the nerve as an external sheath
ii. Inner epineurium is composed of loose connective tissue and serves to cushion the fascicles from trauma. Surrounds group of fascicles.
iii. The substance that is sutured together in epineural repair.
e. Mesoneurium
i. Outermost layer of connective tissue analogous to the mesentery of the intestine
ii. Contains the segmental blood supply of the nerve and is continuous with the epineurium
4. Blood supply
a. Vasa nervorum are small vessels organized segmentally as longitudinal plexi within the epineurium and mesoneurium
b. Extrinsic vessels provide additional nutritional support to the capillaries located within the perineurium and endoneurium
C. Nerve topography
1. Can be categorized based on the number of fascicles within the nerve
a. Monofascicular (e.g., terminal branch): One fascicle, either pure motor or pure sensory
b. Oligofascicular (e.g., common digital nerve): 2 to 10 fascicles
c. Polyfascicular (e.g., radial nerve): >10 fascicles
2. The fascicular organization of the major upper extremity nerves has been mapped including detailed patterns of the motor and sensory nerve fibers, forming the foundation for fascicular and group fascicular repair
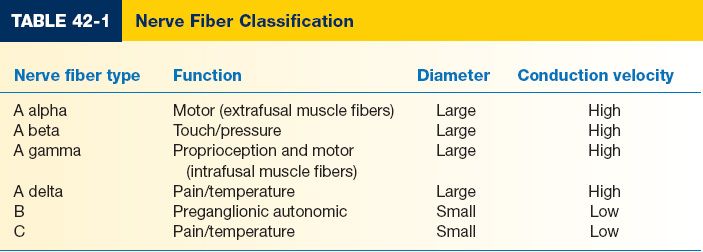
D. Nerve fiber classification: Based on diameter of the nerve fiber (Table 42-1)
1. A Group: Large diameter, high conduction velocity, and myelinated
2. B Group: Small diameter, low conduction velocity, and myelinated
3. C Group: Small diameter, low conduction velocity, and unmyelinated
II. CHRONOLOGY OF NERVE INJURY
A. Nerve degeneration
1. Wallerian degeneration occurs at and distal to the site of nerve injury (may also involve up to 2 cm of the proximal stump)
2. Macrophages invade and clear debris from the axonal tract
3. Neuronal cell body swells and increases protein synthesis to rebuild its injured axon
4. Chromatolysis, or dissolution of the Nissl bodies and peripheral migration of the nucleus, occurs in response to nerve injury or ischemia
1. Injured peripheral nerve fibers can regenerate, provided continuity with the distal portion of the axonal tract is maintained or is reestablished surgically
2. Proliferation of Schwann cells and realignment of the remaining connective tissue into an endoneurial tube occurs (Bands of Büngner), which helps guide the regenerating axon
3. Within 24 hours post-injury, a growth cone is formed at the proximal nerve segment containing an abundance of axonal sprouts
4. Neurotrophic growth factors and chemotactic agents guide the regenerating axons to either motor end plates or sensory end organs
5. Regenerating axons demonstrate neurotropism, or an affinity for neural tissue with end-organ specificity
6. If nerve disruption and/or scarring is severe, regenerating axons cannot cross the gap, and regeneration does not occur
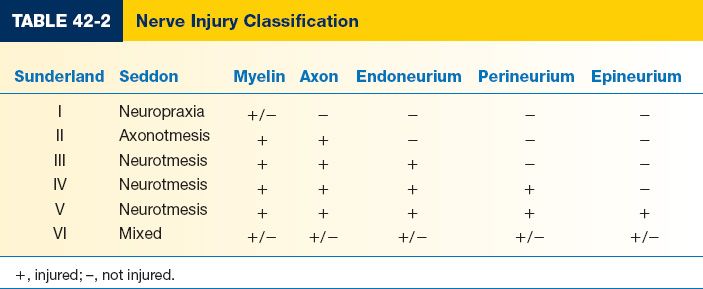
III. CLASSIFICATION OF NERVE INJURY (TABLE 42-2)
A. *Seddon: Introduced in 1947, based on degree of nerve fiber damage
1. Neuropraxia: Local transient block in nerve conduction with minimal axonal damage
a. Anatomic continuity is preserved
b. Wallerian degeneration does not occur
c. Recovery is a few days or weeks
2. Axonotmesis: Severe axonal damage occurs within the nerve
a. Anatomic continuity is preserved
b. Wallerian degeneration occurs
c. Recovery is a few months
3. Neurotmesis: Nerve is transected
a. Anatomic continuity is lost
b. Wallerian degeneration occurs
c. Recovery is never complete, and best outcomes are achieved with nerve repair
B. Sunderland (with Mackinnon modification): Introduced in 1951, expanding Seddon classification and later modified by Mackinnon
1. *First-degree injury: Same as Seddon neuropraxia
2. *Second-degree injury: Same as Seddon axonotmesis
3. *Third-degree injury: Myelin, axon, and endoneurium are disrupted, and recovery varies from almost complete to no recovery whatsoever
4. *Fourth-degree injury: Perineurium is disrupted in addition to third-degree injury findings, and nerve regeneration is prevented by scar tissue at the site of injury. Also known as a neuroma-in-continuity.
5. *Fifth-degree injury: Same as Seddon neurotmesis, where the nerve is transected and no functional recovery is expected
6. *Sixth-degree injury: Nerve injury results in mixed recovery due to varying degrees of pathology along the length of the nerve and from fascicle to fascicle
A. After a thorough history is obtained, it is absolutely necessary to accurately document all motor and sensory deficits, as well as normal findings on neuromuscular examination
1. Allows the clinician to formulate an appropriate treatment plan
2. Allows the clinician to monitor recovery of function and will guide decision-making on whether to operate versus continue observation
B. Neuromuscular examination
1. Motor
a. Signs of motor deficit include loss of function, weakness, and muscular atrophy
b. Often difficult to obtain in the obtunded or inebriated patient and, therefore, should be repeated as soon as the patient’s mental status improves
c. Be aware that certain anatomic anomalies can mask the site of nerve injury
i. *Martin–Gruber anomaly: Motor connections from the median nerve cross over to the ulnar nerve in either the proximal forearm (from the median nerve) or the distal forearm (from the anterior interosseus nerve)
ii. *Riche–Cannieu anomaly: Motor connections between the recurrent motor branch of the median nerve and the deep branch of the ulnar nerve in the palm
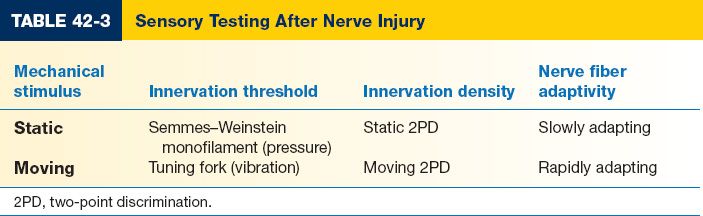
2. Sensory (Table 42-3)
a. Signs of sensory deficit include loss of sensation, uncoordinated fine motor control due to loss of graded somatosensory feedback, and flattening of dermal ridges
b. Cutaneous mechanoreceptors
i. Provide the senses of touch, pressure, and vibration
ii. All innervated by A beta fibers
iii. Slowly adapting type I mechanoreceptors perceive form and roughness, have small receptive fields, and produce sustained firing rates to static stimulation
iv. Slowly adapting type II mechanoreceptors perceive skin stretch, have large receptive fields, and produce sustained firing rates to static stimulation
v. Rapidly adapting mechanoreceptors perceive flutter and slippage, have small receptive fields, and produce a transient firing pattern at the onset and offset of the mechanical stimulus
vi. *Pacinian corpuscles perceive high-frequency vibration, have large receptive fields, and produce a transient firing pattern at the onset and offset of the mechanical stimulus
c. Tinel’s sign
i. Lightly percussing over the distal end of the proximal nerve segment elicits paresthesias
ii. Presence of Tinel’s sign indicates that growth cones are attempting regeneration. Advancing Tinel’s sign is an indicator of active nerve regeneration.
d. Two-point discrimination (2PD)
i. A measurement of innervation density
ii. Static 2PD is normal up to 6 mm
iii. Moving 2PD is normal up to 3 mm
e. Tuning fork (vibration)
i. A measurement of innervation threshold
ii. Use a 128-Hz tuning fork placed over a bony prominence
f. Semmes–Weinstein monofilaments (pressure)
i. A measurement of innervation threshold
ii. The monofilament delivers a constant pressure directly proportional to its stiffness and is designed to bend at a predefined weight
C. Electrodiagnostic studies
1. Evaluates the electrophysiological health of motor and sensory nerves, and their effector organs (e.g., muscle, sensory end organs)
2. Serves as a diagnostic adjunct to managing peripheral nerve injuries
3. Nerve conduction studies
a. An electrical stimulus in the range of 20 to 100 V is applied proximally to the nerve for 0.1 ms
b. Compound muscle action potentials (CMAPs) or sensory nerve action potentials (SNAPs) are then recorded distally from muscle or terminal cutaneous sensory branches, respectively, and represent the sum of all action potentials produced in an individual motor or sensory nerve
c. Amplitude of the action potential is a function of the number of axons that are depolarized by the electrical stimulus and is the height of the negative peak from baseline or the difference between the negative and positive peaks
d. Latency of the action potential is the delay or time between the onset of the electrical stimulus and the onset of the negative peak
e. Conduction velocity is the rate by which an action potential propagates down the nerve and is influenced by nerve diameter and the extent of myelination
f. Three pathologic mechanisms affect peripheral nerve injuries
i. Axonal degeneration manifests as reduced amplitude
ii. Demyelination manifests as reduced conduction velocity
iii. Conduction block demonstrates no conduction across the region of abnormality, but normal conduction distally
4. Electromyography
a. Either a needle is inserted into muscle or surface electrodes are used on the skin to record electrical activity
b. Recordings are made at rest, with needle insertion, and with voluntary muscle contraction
i. Normal muscle is electrically silent at rest
ii. Needle insertion produces a brief characteristic burst known as insertional activity
iii. During voluntary muscle contraction, motor units fire repetitively with a frequency proportional to the amount of effort exerted
c. A motor unit is a single A alpha neuron and all the muscle fibers innervated by it
i. The sum of all action potentials produced by the muscle fibers within a motor unit is known as the motor unit action potential (MUAP)
ii. The MUAP amplitude, duration, and firing pattern are typically recorded and correlate with overall muscle health
d. Spontaneous firing of individual muscle fibers at rest is abnormal and represents denervation of the muscle
i. Fibrillations are spontaneous subclinical contractions of individual muscle fibers
ii. Fasciculations are involuntary contractions of muscle fiber groups (fascicles) or of the entire muscle
iii. Myotonia is the delayed relaxation of muscle after a contraction
iv. While fibrillations can be detected on electromyography, both fasciculations and myotonia are clinically noted
e. Myopathic disease results in shorter duration and lower amplitude of MUAPs, and a decrease in the number of motor units
f. Neuropathic disease demonstrates poor motor unit recruitment with increasing effort
g. Reinnervated muscle demonstrates MUAPs with higher amplitudes and longer durations due to an increased number of muscle fibers per motor unit
h. Denervated muscle will fibrillate with positive sharp waves that usually appear 2 to 3 weeks after axonal loss
V. GENERAL PRINCIPLES OF NERVE REPAIR, TRANSFER, AND GRAFTING
A. Timing of nerve repair
1. Reconstruction of motor nerves is performed when reinnervation is expected before complete muscle atrophy, and if the muscles supplied by the nerve are not injured themselves
a. *Nerves regenerate at a maximal rate of 1 mm per day (1 inch per month)
b. Muscle atrophy begins immediately and little recovery is expected if nerve repair occurs later than 18 to 24 months postinjury
c. Unlike motor nerves, there is no time limit for reinnervation of sensory end organs, although protective sensation may only be achieved
2. It is important to prioritize reconstruction in the case of multiple nerve injuries and proximal nerve injuries
a. Example: Recovery of meaningful intrinsic hand function after reconstruction of an adult brachial plexus injury is unlikely because of the time and distance required for reinnervation
b. Reconstruction should focus on reinnervating the proximal musculature to restore shoulder abduction and elbow flexion, as well as providing protective sensation to the ulnar aspect of the hand
3. Immediate primary repair of a sharply transected nerve is associated with the best functional recovery
a. Nerve exploration should proceed within the first 72 hours postinjury, to avoid depletion of neurotransmitters from the distal nerve segment
b. *After 72 hours, the distal nerve segment will no longer respond to direct electrical stimulation and becomes exceedingly difficult to identify intraoperatively within the wound bed
4. If the nerve is stretched, crushed, avulsed, or blasted, the zone of injury often extends a considerable distance both proximally and distally beyond the site of transection
a. Immediate primary repair should be avoided
b. In the acute setting, the proximal and distal nerve segments should be sutured together to prevent retraction
c. Once the wound is stable with no infection (usually 3 weeks), all of the scarred tissue including any diseased nerve is excised
d. Definitive reconstruction often requires use of a nerve graft
5. For closed nerve injuries, the patient should be followed closely for recovery of function
a. Electrodiagnostic studies should be obtained early to determine baseline values (within the first 4 to 6 weeks)
b. Electrodiagnostic studies should then be repeated at 12 weeks
i. If incomplete recovery, continue to observe patient with periodic electrodiagnostic studies and neuromuscular examinations
ii. If no clinical or electrical signs (absence of MUAPs) of recovery are evident, then nerve exploration is warranted
1. The procedure of choice when there is no nerve gap
2. Goals of nerve repair
a. Properly align the nerve ends, often guided by fascicular anatomy and vascular landmarks
b. Use the fewest number of sutures possible to minimize bulk and foreign body response
c. Trim the nerve ends to remove scar, hemorrhage, and protruding fascicles (which lie outside the plane of the epineurium due to normal endoneurial pressure)
d. Coapt the nerve ends with minimal tension at the repair site
e. Sometimes the proximal and distal nerve segments can be mobilized to gain additional length to facilitate nerve repair and avoid nerve grafting (e.g., up to 3 cm of length can be gained when the ulnar nerve is transposed anterior to the medial epicondyle)
3. Types of nerve repair
a. Epineurial repair
i. *Commonly used for digital nerves
ii. Advantages include shorter operative time, less traumatic with no violation of intraneural contents, and technically easier
iii. Disadvantages include may not ensure proper fascicular alignment and tension on repair site from tendency of nerve ends to retract
b. Fascicular (perineurial) repair
i. Commonly used for nerves with fewer than five fascicles and for nerve grafting
ii. Main advantage is maximal control over fascicular alignment
iii. Disadvantages include longer operative time, more traumatic, and technically demanding
c. Group fascicular repair
i. Indicated when the topography of the nerve is clearly defined and when motor and sensory branches are readily identifiable within the main trunk (e.g., median nerve 5 cm proximal to the wrist, ulnar nerve 7 to 8 cm proximal to the wrist)
ii. Intraoperative awake stimulation and histochemical evaluation of motor (acetylcholinesterase) and sensory (carbonic anhydrase) axons can be performed to aid fascicular identification
d. The superiority of one nerve repair type over another has not been clearly established
C. Nerve transfer
1. Goal: Is to convert a proximal nerve injury into a distal nerve injury by sacrificing a less important redundant nerve in order to reconstruct a more important nonfunctioning nerve close to its effector organ favoring earlier reinnervation
2. Indication: Brachial plexus injuries, proximal nerve injuries, delayed presentation, segmental loss, and scarred wound bed
3. If the distal nerve segment is unavailable, direct muscle implantation (neurotization) may allow for some return of motor function
D. Nerve grafting
1. The procedure of choice when a nerve gap is present
2. Types of nerve grafts
a. Autografts
i. Gold standard against which all other nerve grafts are compared
ii. Indicated when primary nerve repair is not possible without producing tension at the repair site
iii. Provide a biologic scaffold containing neurotrophic factors and viable Schwann cells supporting axonal regeneration
iv. Vascularized autografts are preferred in scarred or radiated wound beds, or when extremely long donor nerves are required
i. Similar to autografts, freshly harvested allografts provide a biologic scaffold that is eventually repopulated by host Schwann cells and axons
ii. Limiting factor: Host immunosuppression; consequently allografting is almost never performed
iii. Currently preferred immunosuppressive agent is tacrolimus (FK506) due to its neuroregenerative potential
3. Commonly used donor nerves
a. Ideal donor nerves are long with minimal branching patterns, and the sensory deficit produced by their harvest should be limited to a noncritical region
b. Donor nerves with multiple branches should be reversed in orientation during inset to minimize loss of regenerating axons through the branches, thereby maximizing the number of regenerating axons that ultimately innervate the end organ
c. Sural nerve
i. Provides 30 to 40 cm of nerve graft
ii. *Located immediately adjacent to the lesser saphenous vein 2 cm posterior to the lateral malleolus and approximately 1 to 2 cm proximal
iii. The nerve is composed of spinal nerve roots from S1 and S2 and is formed from branch of both the common peroneal and posterior tibial nerves.
iv. In the posterior calf, the sural nerve emerges from between the two heads of the gastrocnemius muscle and runs with the small saphenous vein inferiorly to curve under the lateral malleolus
v. In the area of the lateral malleolus, the nerve divides into several branches that run over the lateral foot. The branching pattern may be variable.
vi. The nerve may be harvested as a vascularized nerve graft within the cutaneous paddle of a fibula free flap and is often identified during skin paddle dissection of the fibula flap
vii. Harvest results in loss of sensation along the dorsolateral foot; generally well-tolerated, but painful neuromas form in 5% of patients
d. Lateral antebrachial cutaneous nerve
i. Provides 5 to 8 cm of nerve graft
ii. Located adjacent to the cephalic vein at the junction of the lateral and middle thirds of the forearm
iii. Harvest results in loss of sensation along the lateral aspect of the forearm
e. Anterior division of the medial antebrachial cutaneous nerve
i. Provides 10 to 20 cm of nerve graft
ii. Located adjacent to the basilic vein at the junction of the middle and medial thirds of the forearm
iii. Harvest results in loss of sensation along the medial aspect of the forearm
f. Posterior interosseus nerve (terminal sensory branch)
i. Provides 2 to 5 cm of nerve graft
ii. *Located in the floor of the fourth extensor compartment
4. Instead of nerve grafting, nerve conduits can be used for short nerve gaps (up to 3 cm) in noncritical, small-diameter sensory nerves
a. Nerve conduits can be biologic (vein, muscle, and decellularized nerve) or nonbiologic (polyglycolic acid, silicone, and polytetrafluoroethylene [PTFE])
b. They function by realigning the regenerating axons in a natural milieu of neurotrophic factors
c. Alternative uses of nerve conduits include forming a protective wrap around a nerve repair site or temporarily holding nerve ends together in large nerve gaps prior to nerve grafting
5. Additional options for reducing a nerve gap and possibly avoiding the need for nerve grafting include nerve transposition, nerve mobilization, and bone shortening
COMPRESSION SYNDROMES
I. PATHOPHYSIOLOGY OF NERVE COMPRESSION
A. Mechanical compression
1. Acute nerve compression causes local ischemia that results in a focal conduction block, which is reversible as long as the duration of compression is brief
2. Increasing pressure on the nerve leads to predictable changes in nerve dynamics
a. 20 mmHg: Reduced epineurial blood flow
b. 30 mmHg: Impaired axonal transport
c. 40 mmHg: Parasthesias
d. 50 mmHg: Epineurial edema
e. 60 mmHg: Complete intraneural ischemia
3. Prolonged nerve compression eventually causes focal demyelination
4. This is followed by subendoneurial and synovial edema, axonal damage, and finally nerve fibrosis
5. Chronic nerve entrapment syndromes usually present with a mixedclinical picture of demyelinating and axonal patterns of injury
B. Traction
1. Entrapment can tether the nerve leading to limited excursion and reduced gliding
2. Limb motion can further cause traction-induced conduction block
C. Double-crush phenomenon
1. A given locus of compression impairs axonal transport along the entire length of the nerve
2. This lowers the threshold or predisposes the nerve to a second locus of compression, which can then become symptomatic (e.g., thoracic outlet syndrome plus carpal tunnel syndrome)
D. Systemic conditions
1. Can depress overall peripheral nerve function, which in turn lowers the threshold for symptoms
2. Examples include diabetes mellitus, alcoholism, hypothyroidism, lysosomal storage diseases, polysaccharidoses, and exposure to industrial solvents
II. MEDIAN NERVE
A. Carpal tunnel syndrome
1. Epidemiology
a. Most common mononeuropathy of the upper limb
b. Caused by mechanical compression of the median nerve in a fixed, rigid space due to idiopathic synovitis of the digital flexor tendons
c. Other less common causes include herniation of a ganglion cyst, hypertrophied lumbrical muscles, anomalous flexor pollicis longus muscle belly, and persistent median artery
d. Intrinsic risk factors include female gender, pregnancy, diabetes mellitus, and rheumatoid arthritis
e. Controversial risk factors include repetitive or forceful tasks, mechanical stress, occupational posture, vibration, and temperature
f. An anatomically small carpal tunnel is not a risk factor
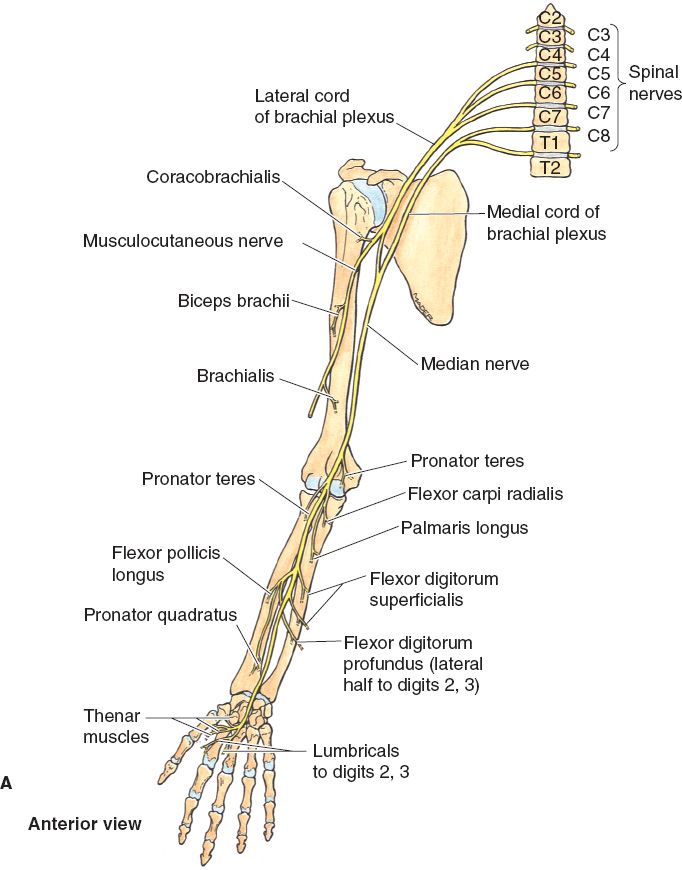
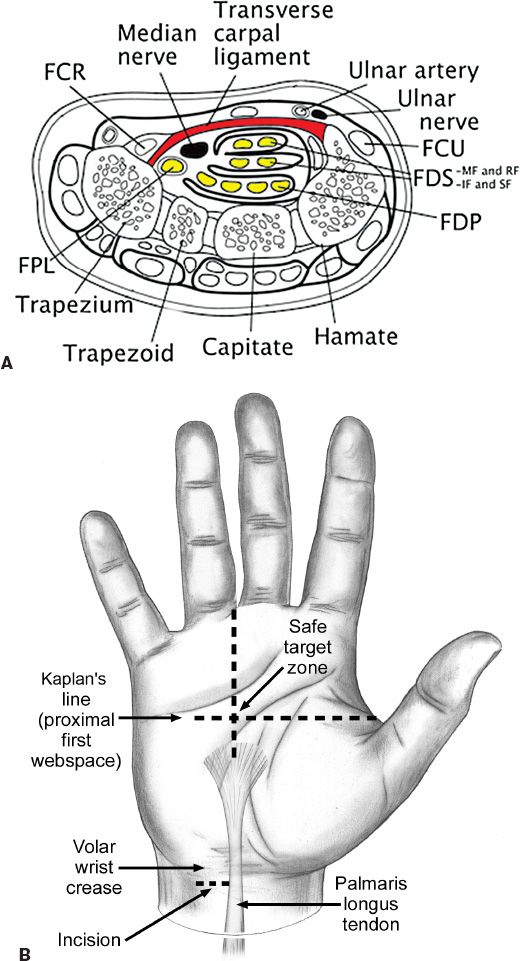
2. Anatomy (Fig. 42-1A)
a. Carpal tunnel (Fig. 42-2) boundaries
i. Radial: Scaphoid tuberosity and trapezium
ii. Ulnar: Pisiform and hook of the hamate
iii. Roof: Transverse carpal ligament
iv. Floor: Carpal bones and volar interosseus ligaments
b. Carpal tunnel contents
i. One nerve: Median nerve
ii. Nine tendons: Flexor pollicis longus (one), flexor digitorum superficialis (four), and flexor digitorum profundus (four)
c. Median nerve branches
i. *Palmar cutaneous branch: Arises 4 to 5 cm proximal to the wrist and provides sensation to the thenar skin
ii. Recurrent motor branch: Usually arises at or just beyond the distal edge of the transverse carpal ligament from the radiopalmar aspect of the nerve, and supplies the thenar musculature and the radial two lumbricals
d. Kaplan’s cardinal line
i. Oblique line from the apex of the interdigital fold between the thumb and index finger toward the hook of hamate and parallel with the proximal palmar crease
ii. Intersection of this line with the axis of the long finger localizes the recurrent motor branch
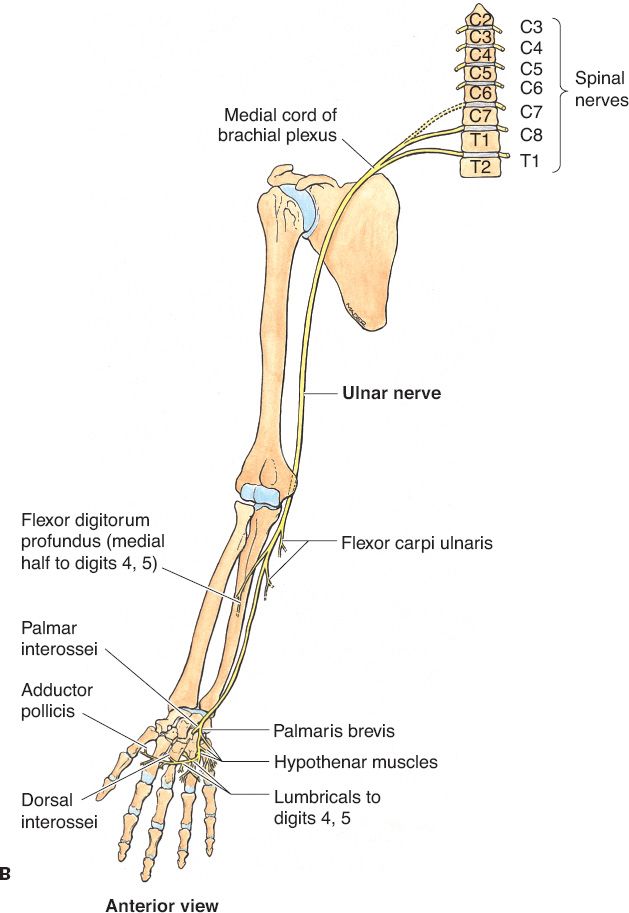
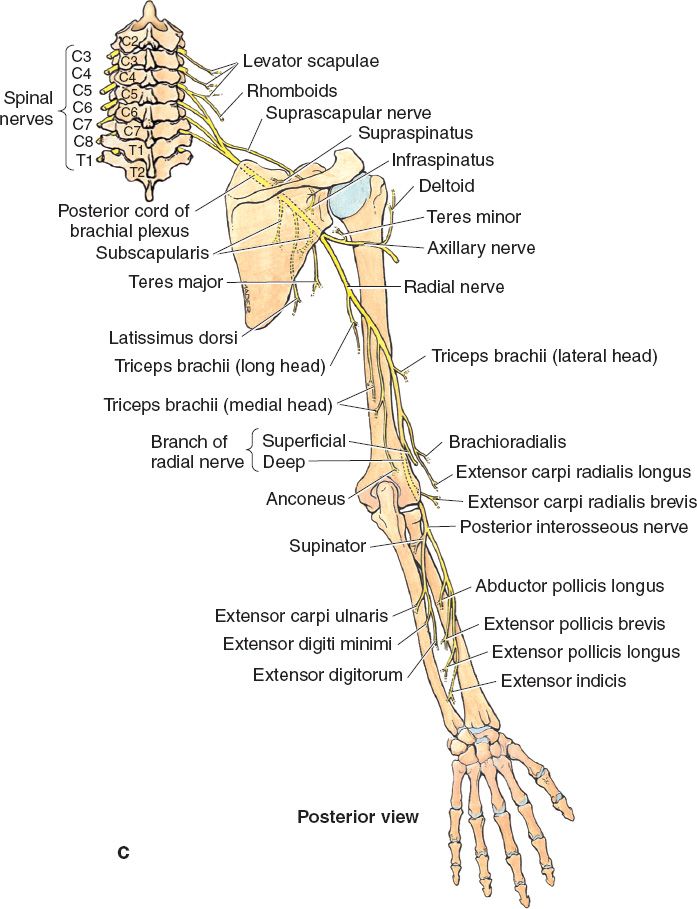
Figure 42-1. Course of median, ulnar, and radial nerves in the upper extremity. A: Course of median nerve. B: Course of ulnar nerve. C: Course of radial nerve. (From Agur AMR, Lee MJ, eds. Grant’s Atlas of Anatomy. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999.)
a. History and neuromuscular examination
i. Pain and parasthesias of the radiopalmar hand, often worse at night and with repetitive movements
ii. The thenar skin is spared of sensory disturbances because it is innervated by the palmar cutaneous branch
iii. Advanced cases may demonstrate thenar wasting
iv. Phalen’s maneuver: The wrist is palmar flexed to 90 degrees and parasthesias are observed in the median nerve distribution of the affected hands within 60 seconds
v. Tinel’s sign: Lightly percussing over the flexor retinaculum elicits parasthesias (less sensitive, but more specific than the Phalen maneuver)
b. Electrodiagnostic studies (highly operator dependent)
i. Motor latencies greater than 4.5 ms
ii. Sensory latencies greater than 3.5 ms
iii. Conduction velocities less than 50 m/s
Figure 42-2. The carpal tunnel. A: Illustration of the cross-sectional anatomy of the carpal tunnel. B: Illustration of the “safe-zone” for a carpal tunnel release. Intersection of Kaplan’s line with ring finger is classically the location of the recurrent branch of the median nerve. (From Berger RA, Weiss AC, eds. Hand Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.)
4. Nonoperative treatment (first line treatment)
a. Splint the wrist in neutral position, and wear continuously or only at night depending on the severity of symptoms
b. Anti-inflammatory agents (e.g., NSAIDs) to reduce inflammation
c. Optimize management of systemic conditions (e.g., diabetes mellitus and rheumatoid arthritis)
d. Steroid injections may offer transient relief in 80% of patients, with better results in milder cases
i. Often beneficial in pregnant patients with debilitating symptoms, or others with transient edema
ii. Response to steroids is also prognostic, predictive of a good response to surgery
5. Operative treatment
a. Open carpal tunnel release
i. Complete release of the transverse carpal ligament through an incision ulnar and parallel to the thenar crease to avoid injury to the palmar cutaneous branch
ii. Provides better exposure, but leaves a longer scar
b. Endoscopic-assisted carpal tunnel release
i. Leaves a shorter scar, but anatomic anomalies may be more difficult to recognize given impaired visualization relative to the open technique
ii. No superiority has been demonstrated over the open technique
c. Synovectomy is only indicated in cases of proliferative or invasive tenosynovitis
d. Internal neurolysis, epineurotomy, and decompression of Guyon’s canal are not indicated routinely in carpal tunnel syndrome
B. Pronator syndrome
1. Sites of compression (from proximal to distal)
a. Proximal ligamentous attachment or accessory origin of the humeral head of the pronator teres (ligament of Struthers)
b. Bicipital aponeurosis (lacertus fibrosis)
c. Between the humeral and ulnar heads of the pronator teres (most common)
d. Proximal edge of the flexor digitorum superficialis arch
2. Diagnosis
a. *Pain in the proximal volar forearm with associated hypesthesia/parasthesias in the median nerve distribution, including involvement of the palmar cutaneous branch (helps differentiate it from carpal tunnel syndrome and anterior interosseus syndrome)
b. Reproducible symptoms with isolated, resisted contraction of the biceps, pronator teres, or flexor digitorum superficialis may indicate the site of compression
c. Electrodiagnostic studies are of limited benefit
3. Treatment
a. Splinting and rest may resolve symptoms in up to 50% of patients
b. If conservative measures fail, then all potential sites of compression above and below the elbow must be explored and released
C. Anterior interosseus syndrome
1. Etiology
a. Probably not a true compression neuropathy, but a shared clinical manifestation or constellation of symptoms among several different causes, including mechanical compression (e.g., anatomic anomaly, forearm mass), inflammatory (e.g., infection, idiopathic), and posttraumatic (e.g., forearm fracture, hemorrhage into the deep musculature)
b. Approximately one-third of cases occur spontaneously
c. The most common site of compression is between the humeral and ulnar heads of the pronator teres
2. Diagnosis
a. Weakness or loss of function of the flexor pollicis longus, flexor digitorum profundus to the index and long fingers, and pronator quadratus
b. *Since the anterior interosseus nerve is purely motor, no sensory symptoms
c. If asked to make an “OK” sign, the patient will make a triangle instead of circle due to lack of flexion of the interphalangeal joint of the thumb and distal interphalangeal joint of the index finger (pinch deformity)
d. Unlike the pronator syndrome, electrodiagnostic studies are a useful diagnostic adjunct
a. Managed similar to a closed nerve injury with baseline electrodiagnostic studies obtained within 4 to 6 weeks
b. Repeat electrodiagnostic studies at 12 weeks and if recovery is still incomplete, continue to observe patient
c. If no clinical or electrical signs (absence of MUAPs) of recovery are evident at 12 weeks, then nerve decompression is warranted
III. ULNAR NERVE (FIG. 42-1B)
A. Cubital tunnel syndrome
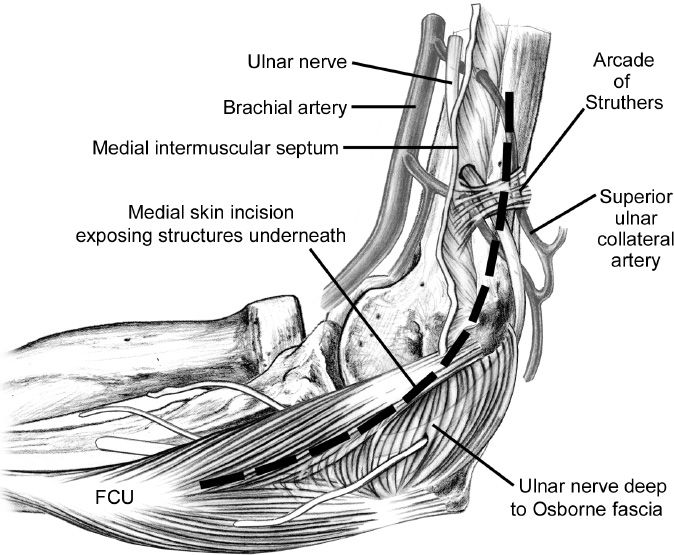
1. Sites of compression (from proximal to distal) (Fig. 42-3)
a. Medial intermuscular septum of the brachium
b. Thick fascial band between the medial head of the triceps and the medial intermuscular septum located 8 cm above the elbow (arcade of Struthers)
c. Cubital tunnel
i. Roof: Aponeurotic expansion of the two heads of the flexor carpi ulnaris (FCU; Osborne ligament)
ii. Floor: Ulnar collateral ligament of the elbow, joint capsule, and olecranon
d. Fascia of the FCU
2. Diagnosis
a. History and neuromuscular examination
i. Hypesthesia/parasthesias of the small and ulnar half of the ring fingers and dorsoulnar hand
ii. Weakness of grip strength and intrinsic wasting in advanced cases
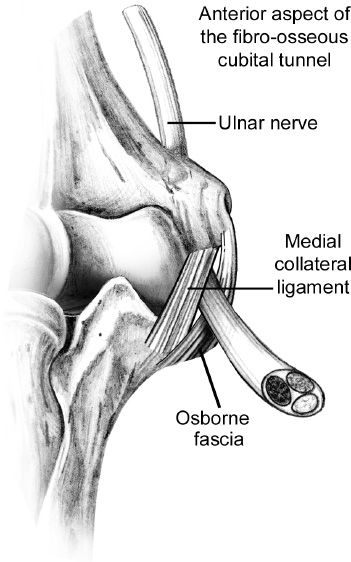
Figure 42-3. Anatomy of the cubital tunnel. FCU, flexor carpi ulnaris. (From Berger RA, Weiss AC, eds. Hand Surgery. Philadelphia, PA: Lippincott; 2004.)
iii. Positive Tinel’s sign over the medial elbow
iv. Possible ulnar nerve subluxation with elbow flexion
v. Phalen analog: Hypesthesia/parasthesias in the ulnar nerve distribution within 60 seconds of full elbow flexion
vi. Scratch collapse test (controversial): With palms facing one another, a patient attempts adduction resistance to an examiner’s efforts. The examiner then scratches the skin over the potential site of compression and attempts to adduct the forearm—sudden, temporary weakness of that forearm is a positive response.
b. Ancillary studies
i. Evidence of denervation in the first dorsal interosseus on electromyography (most common)
ii. Abductor pollicis brevis should be normal (this excludes a C8/T1 nerve root or plexus lesion)
iii. Obtain elbow plain films if history of trauma or abnormal range of motion
3. Operative treatment
a. In situ decompression: Procedure of choice given similar outcomes with transposition techniques
b. Ulnar nerve transposition (subcutaneous, submuscular, or intramuscular): Indicated in recurrent cases of cubital tunnel syndrome and patients with ulnar nerve subluxation
e. Medial epicondylectomy: Useful in post-traumatic cases with bony deformity, but carries the risk of damaging the ulnar collateral ligament leading to elbow instability
B. Ulnar tunnel syndrome (compression of ulnar nerve in Guyon’s canal)
1. Etiology
a. Ganglion cyst (most common)
b. Muscle anomalies
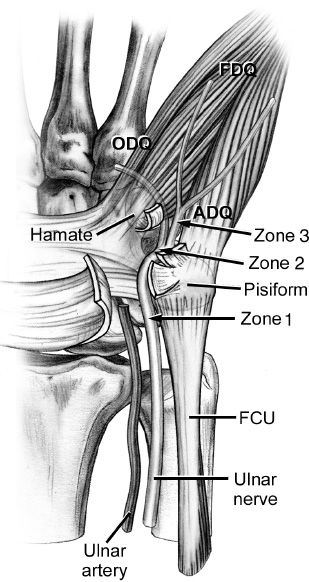
Figure 42-4. Anatomy of Guyon’s canal at the wrist. Zone 1: ulnar nerve, motor and sensory. Zone 2: deep motor branch. Zone 3: superficial sensory branch. ADQ, abductor digiti quinti; FCU, flexor carpi ulnaris; FDQ, flexor digiti quinti; ODQ, opponens digiti quinti. (From Berger RA, Weiss AC, eds. Hand Surgery. Philadelphia, PA: Lippincott; 2004.)
c. Thrombosis or pseudoaneurysm of the ulnar artery (hypothenar hammer syndrome)
d. Hook of the hamate fracture
e. Edema/scarring from burns
f. Inflammatory arthropathy
2. Anatomy (Fig. 42-4)
a. Guyon’s canal boundaries
i. Radial: Hook of the hamate
ii. Ulnar: Pisiform
iii. Roof: Volar carpal and pisohamate ligaments
iv. Floor: Transverse carpal ligament
b. Guyon’s canal contents
i. One artery: Ulnar artery
ii. One nerve: Ulnar nerve
iii. The ulnar nerve is located ulnar to the ulnar artery
c. Guyon’s canal zones
i. Zone 1: Proximal to the ulnar nerve bifurcation
ii. Zone 2: Contains the deep motor branch of the ulnar nerve
iii. Zone 3: Contains the superficial sensory branch of the ulnar nerve
d. Ulnar nerve branches
i. Dorsal sensory branch: Arises 4 to 5 cm proximal to the pisiform and provides sensation to the dorsoulnar hand
ii. Deep motor branch: Arises within Guyon’s canal (more radial) and supplies the intrinsic musculature
iii. Superficial sensory branch: Arises within Guyon’s canal (more ulnar) and provides sensation to the small and ulnar half of the ring fingers
a. Pain in the ulnar wrist with hypesthesia/parasthesias in the small and ulnar half of the ring fingers
b. *The dorsoulnar hand is spared of sensory disturbances because it is innervated by the dorsal sensory branch
c. Positive Tinel’s sign over Guyon’s canal
d. Symptoms are exacerbated by sustained hyperextension or hyperflexion of the wrist
e. Ulnar “Paradox”: More likely to get clawing with distal compared to proximal ulnar nerve compression due to sparing of the flexor digitorum profundus.
f. A bruit may be present
g. Electrodiagnostic studies are a useful diagnostic adjunct
4. Nonoperative treatment
a. Indicated if no identifiable lesion is present
b. Splint the wrist in neutral position
c. Anti-inflammatory agents (e.g., NSAIDs) to reduce inflammation
d. Activity modifications
5. Operative treatment
a. Indicated if an identifiable lesion is present or failure of conservative measures
b. Open ulnar tunnel release
i. Complete release of the volar carpal and pisohamate ligaments
ii. Divide the fibrous arch near the origin of the hypothenar musculature
iii. Explore the floor of Guyon’s canal for masses and fractures
iv. Examine the ulnar artery with the tourniquet up and then down
IV. RADIAL NERVE (FIG. 42-1C)
A. Posterior interosseus syndrome
1. Sites of compression (from proximal to distal)
a. Thickened fascial tissue superficial to the radiocapitellar joint
b. Recurrent vessels of the radial artery (leash of Henry)
c. Fibrous bands within the extensor carpi radialis brevis
d. Proximal edge of the supinator (arcade of Frohse, most common)
e. Distal edge of the supinator
2. Diagnosis
a. History and neuromuscular examination
i. Gradual weakness of finger and wrist extensors
ii. Similar to anterior interosseus syndrome where motor deficit is the primary issue with lack of sensory complaints
iii. Acute onset after trauma
iv. Rheumatoid disease at the elbow can mimic symptoms
v. *Incomplete syndrome may be confused for tendon rupture (check for tenodesis)
b. Ancillary studies
i. Electrodiagnostic studies are a useful diagnostic adjunct
ii. Elbow plain films to rule out radial head dislocation or fracture
iii. Magnetic resonance imaging or ultrasound if there is concern for a soft tissue mass
3. Treatment
a. Managed similar to a closed nerve injury with baseline electrodiagnostic studies obtained within 4 to 6 weeks
b. Repeat electrodiagnostic studies at 12 weeks and if recovery is still incomplete, continue to observe patient
c. If no clinical or electrical signs (absence of MUAPs) of recovery are evident at 12 weeks, then nerve exploration is warranted
d. Other indications for nerve exploration include post-traumatic (e.g., proximal radius fracture) or if an identifiable lesion is present
e. Steroid injections may be of some benefit in patients with rheumatoid disease
1. Anatomy
a. Radial tunnel boundaries
i. Roof: Extensor carpi radialis longus and brevis
ii. Floor: Radiocapitellar joint capsule proximally, and the biceps tendon and deep head of the supinator distally
b. Radial tunnel contents
i. One nerve: Radial nerve
ii. It runs approximately 5 cm in length from the radiocapitellar joint to the distal edge of the supinator
c. Radial nerve branches
i. Superficial branch: Provides sensation to the dorsoradial hand
ii. Deep branch: Supplies motor innervation to the finger and wrist extensors
2. Diagnosis
a. *Patients primarily complain of pain (weakness is secondary)
b. Pain is located at the lateral elbow and is exacerbated by resisted supination
c. Often related to a work setting consisting of repetitive forceful elbow extension and forearm rotation
d. Must differentiate from lateral epicondylitis (tenderness is more distal in radial tunnel syndrome)
e. Middle finger test: Resisted middle finger extension produces pain in the proximal forearm
f. Electrodiagnostic studies are of limited benefit
g. Steroid injections are both diagnostic and prognostic, predictive of a good response to surgery
3. Treatment
a. Conservative measures are the mainstay of treatment including rest, splinting, steroid injections, and anti-inflammatory agents (e.g., NSAIDs)
b. Nerve exploration is only indicated if conservative measures fail
c. No progression to muscle palsy has ever been documented
C. Wartenberg syndrome
1. Definition: Compression of the superficial branch of the radial nerve
2. Etiology
a. *The superficial branch of the radial nerve becomes subcutaneous 9 cm proximal to the radial styloid between the brachioradialis and extensor carpi radialis longus tendons
b. Many different causes of compression including external (e.g., watch, handcuffs), overuse/repetitive activity (e.g., using a screwdriver), posttraumatic (e.g., wrist contusion), and scissoring of the brachioradialis and extensor carpi radialis longus tendons
3. Diagnosis
a. *Pain and parasthesias over the dorsoradial hand that is exacerbated by wrist movement, index-thumb pinch, or forceful pronation of the forearm
b. May get a false-positive with Finkelstein’s test (pain with ulnar deviation of the wrist with the thumb grasped in the palm)
c. Diagnosis can be confirmed by tracing Tinel’s sign or performing a diagnostic nerve block
d. Electrodiagnostic studies are of limited benefit
4. Treatment
a. Conservative measures are the mainstay of treatment including rest, splinting, steroid injections, and anti-inflammatory agents (e.g., NSAIDs)
b. Nerve exploration is only indicated if conservative measures fail
V. THORACIC OUTLET SYNDROME
A. Epidemiology
1. Represents a common clinical manifestation or constellation of symptoms among several different causes
a. Neurologic: 95% (e.g., brachial plexus)
b. Venous: 3% to 4% (e.g., subclavian vein)
c. Arterial: 1% to 2% (e.g., subclavian artery)
2. Three times more common in women than in men
3. Usually arises between the third and sixth decades of life
4. May be associated with occupations that involve awkward or static arm positioning at or above the shoulder level (e.g., painters and nurses)
5. Paget–Schroetter disease: Sudden, effort-induced thrombosis of the upper extremity deep venous system
B. Sites of compression
1. Interscalene triangle: Between the anterior and middle scalene and the first rib
2. Costoclavicular triangle: Between the clavicle and the first rib
3. Congenital fibromuscular bands: More common in neurologic cases
4. Cervical ribs: Present in 0.5% of the general population and more common in arterial cases (50% to 80% are bilateral)
C. Diagnosis
1. History and neuromuscular examination
a. Pain or dull ache of insidious onset in the shoulder, upper back, and neck (easy fatigability and nighttime pain are common)
b. If neurologic involvement, may have parasthesias
c. If venous involvement, may have visible engorged collateral veins
d. If arterial involvement, may have claudication symptoms
2. Provocative tests
a. Positive response is if the symptoms are reproduced or there is loss of the radial pulse
b. All provocative tests lack sufficient sensitivity and specificity to make the diagnosis with any degree of certainty
c. Adson test: With the affected arm in the dependent position, turn the head to the ipsilateral shoulder, hyperextend the neck, and breathe deeply
d. Halstead (costoclavicular) test: With the affected arm in the dependent position, move the shoulder down and back with the chest out
e. Wright (hyperabduction) Test: With the affected arm externally rotated and abducted 180 degrees, breathe deeply
f. Cervical rotation lateral flexion test: Rotate the head away from the affected arm, then flex the head toward the affected arm (positive response is bone blocking lateral flexion)
3. Ancillary studies
a. Cervical and chest plain films looking for bony abnormalities, cervical ribs, and lung masses (Pancoast tumor)
b. Vascular studies including Doppler or arteriogram/venogram (gold standard, but invasive)
c. Magnetic resonance imaging and electrodiagnostic studies are of limited benefit
d. Attempt diagnostic nerve block with injection into the scalenes
D. Treatment
1. Conservative measures should be attempted first including postural training, stretching/strengthening exercises, activity modification, and anti-inflammatory agents (e.g., NSAIDs)
2. Indications for operative intervention include failure of conservative measures, intractable pain, significant neurologic deficit, and impending or acute vascular catastrophe
3. Operative treatment focuses on removing any potential sites of compression (e.g., cervical rib, release or excision of anterior and middle scalenes, neurolysis of the brachial plexus as indicated)
4. If venous obstruction, consider thrombectomy
5. If arterial obstruction, consider thromboendarterectomy, resection and interpositional graft, or bypass
6. Complications include brachial plexus injury, hemothorax, pneumothorax, chylothorax, and causalgia
TENDON TRANSFERS
I. PRINCIPLES
A. Definition: The relocation of a tendon from a functioning muscle to replace an injured or non-functional muscle-tendon unit
B. Concept of “muscle balance operation”: Tendon transfer (TT) is a redistribution of power units from areas of lesser functional need to areas of greater functional need
C. Developed in the late 1800s as a way to reconstruct the longstanding effects of polio
D. Loss of a single major nerve (i.e., ulnar/median/radial) is more amenable to TT; if two or three nerves are damaged, severe extremity impairment is inevitable
E. Tenets of TT
1. Joints affected need to have a good passive range of motion; joint contractures need to be either prevented or repaired
2. Soft tissue coverage has to be supple and stable—“tissue equilibrium”—TT through scar tissue do poorly and any soft tissue reconstruction should be done prior to TT
3. Simpler procedures have better results; that is, never introduce more than one change of direction in a tendon
4. Each transfer should have the goal of one function
II. INDICATIONS
A. Nerve injury: The most common indication. TT is often needed when nerve injuries are devastating or proximal, as reinnervation will not occur in time to salvage motor units.
B. Muscle/tendon destruction: may be from trauma or disease processes such as rheumatoid arthritis
C. Spastic disorders: A less common indication, but can decrease spasticity and ease hand positioning and hygiene
III. PREOPERATIVE PLANNING
A. Evaluation
1. Establish patient goals/needs
2. Rank priority of the functions desired
3. Assess expectations, making sure they are realistic
4. Verify motivation and ability to follow through with rehabilitation
B. Timing
1. Factors in the recovery of nerve function
a. Children do better than adults who do better than the elderly in recovering nerve function
b. Clean, sharp cuts recover better than large and/or contaminated wounds
c. Injuries close to the muscle have better functional recovery than those far from the target muscle
2. Immediate TT: Only done if there is no chance of neurological recovery; that is, the muscle is destroyed or large section of the nerve is missing and repair/regrowth is not feasible
3. Delayed TT: Usually performed 9 to 12 months following injury to allow for potential regrowth of the nerve before proceeding with TT. The higher the nerve injury, the longer regrowth will take
C. Wound site factors which must be addressed before a TT
1. The bony skeleton must be stabilized
2. The wound surface must be healed or closed
3. Scars must be soft, or must be excised
4. Adequate soft tissue must be present to protect the TT
5. Joint mobility must be maximized for the best result
D. Donor tendon/muscle evaluation
1. Donor muscle assessment: inventory all muscles and rate their power. Only donor muscles with British Medical Research Council (MRC) power grades of 4 or 5 should be used for TT.
a. 0: No active movement
b. 1: Visible muscle movement but not at joint
c. 2: Can create motion at joint, but against gravity
d. 3: Can overcome gravity, but not added resistance
e. 4: Can overcome resistance, but weaker than “normal”
f. 5: Normal strength
2. Control: Tendons to be transferred should have independent power (e.g., the flexor digitorum profundus (FDP) tendon slips do not have independent function and therefore are poor donor choices)
3. Excursion (amplitude)
a. Specific excursions
i. Digits—70 mm
ii. Wrist—30 mm
b. Tenodesis can increase excursion by approximately 25 mm
c. Need to match the donor being transferred with the amount of excursion needed (e.g., a wrist flexor will not work as digit extensor)
4. Need to match strength (e.g., FCU too strong for use as motor for APB). Therapy can improve muscle strength, but not excursion.
5. Location: Reroute the donor tendon in as direct line as possible; do not change direction of tendon more than once
6. Synergism: If possible, use muscles that naturally work together (i.e., wrist extensors and finger flexors); this makes post-op rehabilitation easier
7. Expendability: Is tendon function worth giving up for the benefit gained at its new location?
IV. TTS FOR SPECIFIC NERVE PALSIES
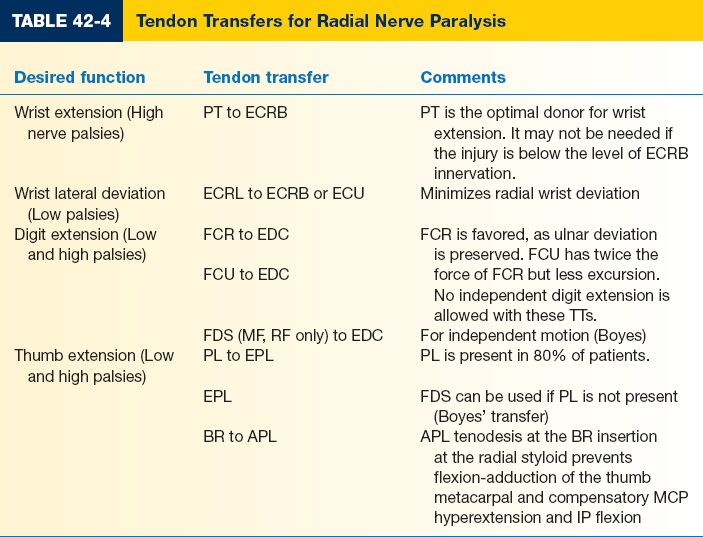
A. Radial nerve palsy
1. Anatomy
a. The radial nerve innervates the triceps, brachioradialis, and ECRL before branching into superficial and deep branches in the forearm
b. The superficial radial nerve is purely sensory, while the deep radial nerve innervates the extensor carpi radialis brevis, supinator, abductor pollicis longus (APL), and all finger extensors
2. Functional deficits
a. High radial nerve paralysis: Wrist, thumb, and digit extension are lost producing a wrist drop deformity. Loss of wrist extension weakens power grip strength. Over time, patients develop an adaptive functional pattern:
i. Patients use wrist flexion to assist with finger extension, that is, the “tenodesis effect”
ii. Wrist flexion may be difficult to overcome later with TTs
iii. Splints should be worn to force wrist extension and assist with finger extension; splints are cumbersome and often tolerated only at night, but critical to eventual outcome
iv. Alternatively, some surgeons advocate doing an end-to-side TT of pronator teres (PT) to ECRB to facilitate grip strength during nerve recovery, termed an “internal splint” procedure
b. Low radial nerve injury: Greater radial wrist deviation than higher level injury due to unopposed ECRL function with loss of other extensors
3. Common TTs for radial nerve paralysis (Table 42-4). Radial nerve palsy TTs require one tendon each for wrist, digit, and thumb extension.
B. Median nerve palsy
1. Nerve function and anatomy
a. The median nerve enters the forearm through the pronator teres, and innervates the PT, flexor carpi radialis (FCR), palmaris longus (PL), and flexor digitorum superficialis (FDS)
b. The anterior interosseous nerve then branches off and innervates the flexor pollicis longus (FPL), pronator quadratus, and radial head of the FDP. Comes off of the median nerve 6-8cm distal to the medial epicondyle.
c. The main branch of the median nerve continues through the carpal tunnel and gives off sensory and motor branches
d. The thenar motor branch innervates the APB, OP, and superficial head of the flexor pollicis brevis (FPB)
e. The common digital branches innervate the lumbricals to the long and index fingers
2. Functional deficits
a. Low median nerve palsy: Loss of nerve function at level of the wrist
i. Loss of thumb opposition from paralysis of APB, OP, and superficial head of the FPB
ii. Loss of the lumbricals to the index and middle fingers
b. High median nerve palsy
i. In addition to the muscle losses above, the anterior interosseous nerve is affected. Patients lose FPL, pronator quadratus, and FDP to index and middle fingers, resulting in loss of thumb and index flexion
ii. Higher level injuries will damage FCR and PT function, but these losses do not require TTs
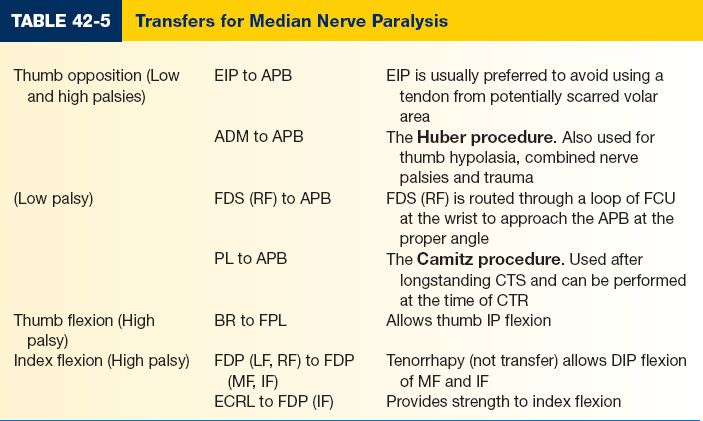
3. Common TTs for median nerve paralysis (Table 42-5)
C. Ulnar nerve palsy
1. Nerve function and anatomy
a. Enters the forearm between the two heads of the FCU, and innervates FCU and the ulnar portion of the FDP
b. Gives off dorsal sensory nerve 7cm proximal to the wrist.
c. Continues into hand via Guyon’s canal, and innervates ADM, FDM, and ODM (i.e., the hypothenar muscles) as well as the seven interosseous muscles, the adductor pollicis, and the ring and small finger lumbricals. The ulnar nerve may innervate part (typically the deep head) or all of the FPB.
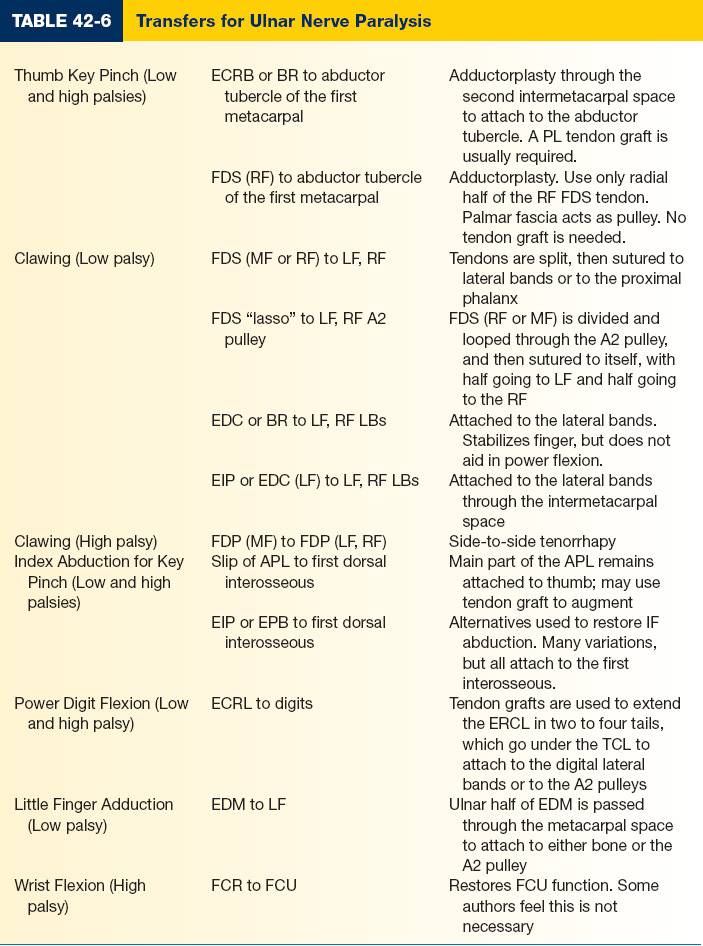
2. Functional deficits
a. Low ulnar nerve palsy
i. Paralysis of AP, the deep head of the FPB, all interossei, hypothenar muscles, and the lumbricals to ring and little fingers
ii. *Clawing of the hand: Specifically, MP hyperextension and IP flexion in the ring and little fingers due to loss of intrinsic musculature in the setting of intact extrinsic function
iii. Weak key pinch: *Due to denervation of the first dorsal interosseous and AP muscles. To compensate, patients use FPL flexion to stabilize the thumb and EPL to adduct the thumb. Exaggerated thumb IP flexion during key pinch is termed Froment’s sign.
iv. Little finger ulnar deviation occurs due to unbalanced extensors (EDC and EDM) to that digit (Wartenberg’s sign)
b. High ulnar nerve palsy
i. *Less clawing than with low ulnar palsies, as the paralysis of the FCU and FDP to ring and little fingers decreases the deforming force
ii. Reconstruction can improve the function of the hand, but normal function usually cannot be restored
3. Common TTs for ulnar nerve paralysis (Table 42-6)
4. Static block procedures can be done to prevent MCP hyperextension, including MCP arthrodesis, MCP joint capsulodeses, or bone blocks on the dorsum of the MCP head. These procedures can be used alone, or in concert with TTs.
V. TTS FOR SPECIFIC DISEASES
A. Rheumatoid arthritis
1. Patients often rupture extensor tendons due to synovial invasion of the tendon or to attrition from a subluxed ulnar head
2. *EDM and little finger EDC are often the first affected due to a prominent ulnar head—caput ulna
a. Treatment
i. Distal EDC tendon from the little finger can be attached to the ring EDC
ii. EIP can be transferred to either the EDM or the EDC
3. If both the ring and little finger EDC tendons have eroded, then either the EIP or the FDS from RF or MF can be used
4. An extensor tenosynovectomy and a Darrach procedure should be done to prevent additional extensor tendon loss
PEARLS
1. It is absolutely necessary to accurately document all motor and sensory deficits, as well as normal findings on neuromuscular examination.
2. Two-point discrimination measures innervation density, while pressure and vibration measure innervation threshold.
3. After 72 hours, the distal nerve segment can no longer be electrically stimulated due to depletion of neurotransmitters.
4. Epineurial repair is equivalent to other repair types in most clinical situations
5. No functional recovery is expected if motor end plates are not reinnervated within 18 to 24 months due to muscle atrophy.
6. In situ decompression is the procedure of choice for most cases of cubital tunnel syndrome.
7. Presence or absence of sensory disturbances in the thenar skin and dorsoulnar hand help differentiate between proximal and distal compressions of the median and ulnar nerves, respectively.
8. Anterior interosseus syndrome and posterior interosseus syndrome produce motor deficits with lack of sensory symptoms.
QUESTIONS YOU WILL BE ASKED
1. With a nerve laceration, how long can one wait until that nerve will no longer be able to be electrically stimulated and produce muscle activity in the OR?
About 3 days.
2. Why is recovery from nerve injuries worse when the injury is more proximal?
Because the nerve fibers must regenerate longer distances, and therefore they require more time to reach their targets. Also, during this time the Schwann cells lose their capacity to support regeneration and the neurons themselves do as well.
3. Is it better to do nerve transfers or tendon transfers for a given deficit?
There is no clear answer, and each case is individualized based on expectations, surgeon preference and experience.
Recommended Readings
Dellon AL. Patient evaluation and management considerations in nerve compression. Hand Clin. 1992;8(2):229–239. PMID: 1613032.
Dvali L, Mackinnon S. Nerve repair, grafting, and nerve transfers. Clin Plast Surg. 2003;30(2):203–221. PMID: 12737353.
Maggi SP, Lowe JB 3rd, Mackinnon SE. Pathophysiology of nerve injury. Clin Plast Surg. 2003;30(2): 109–126. PMID: 12737347.
Mazurek MT, Shin AY. Upper extremity peripheral nerve anatomy: current concepts and applications. Clin Orthop Relat Res. 2001;(383):7–20. PMID: 11210971.
Oates SD, Daley RA. Thoracic outlet syndrome. Hand Clin. 1996;12(4):705–718. PMID: 8953290.
Sammer DM, Chung KC. Tendon transfers: part I. Principles of transfer and transfers for radial nerve palsy. Plast Reconstr Surg. 2009 May;123(5):169e-177e. PMID: 19407608.
Sammer DM, Chung KC. Tendon transfers: Part II. Transfers for ulnar nerve palsy and median nerve palsy. Plast Reconstr Surg. 2009 Sep;124(3):212e-21e. PMID: 19730287.
< div class='tao-gold-member'>