Fig. 12.1
NSF patients exhibited cutaneous thickening and hardening, chiefly on the extremities. (Reprinted from the Lancet, Vol 356(9234), Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients, Pages 1000-1, 2000, with permission from Elsevier)
Following this initial report, the investigators partnered with the Centers for Disease Control (CDC) to create a case definition, ultimately resulting in the recognition of eight additional cases with a similar constellation of symptoms [2]. Although all of the patients identified in the original cohort were receiving hemodialysis at the time of disease onset, this expanded group included some patients receiving hemodialysis, some receiving peritoneal dialysis, and a few patients with chronic kidney disease (CKD) who had never before been dialyzed [2].
In 2001, the disease was named nephrogenic fibrosing dermopathy (NFD) accounting for (1) the characteristic non-inflammatory cutaneous fibrosis and its exclusive occurrence (2) in the setting of severe kidney disease [2]. Although the cause of NSF would not be determined until 2006, this defining paper hypothesized a possible infectious and/or toxic etiology in light of its rapid, recent emergence and clustering around dialysis centers [2].
In 2003, the first NFD autopsy was performed [3]. In addition to cutaneous fibrosis, the findings of significant fibrosis of the diaphragm, psoas muscle, renal tubules and testes underscored the systemic nature of the process [3]. The disease was renamed “nephrogenic systemic fibrosis” to better reflect the extent of the disease process (Fig. 12.2).
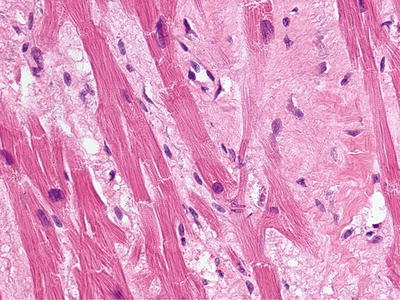

Fig. 12.2
Fibrosis involving the heart of a deceased NSF patient. (With permission of the International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR.org))
That same year, a chart review of cases present in the International NSF Registry (icnsfr.org) demonstrated a preponderance of certain comorbidities in patients with NSF. Patients had a greater likelihood of hypercoagulability, and increased rates of thrombosis and vascular surgery were noted in the days to weeks preceding NSF onset. They also seemed to display a disproportionately high brain tumor incidence than would be expected among patients with ESRD or CKD [4]. This, in addition to continued geographic clustering, strengthened the hypothesis that a toxic exposure could be fueling NSF [4–6].
In the fall of 2005, a lecture was presented at the New England Dermatologic Society meeting at Yale University, during which a strong correlation between imaging studies just prior to the onset of NSF was described—publicly implicating GBCAs as the suspected etiopathogenic trigger of NSF [7].
In January 2006, Grobner et al. published the first medical report implicating GBCAs as the potential cause of NSF [8]. This study included a case series of nine ESRD patients who developed NSF within 3 months following GBCA administration for MRA studies [8]. This observation was further substantiated in a paper from Denmark published in November 2006 describing a similar temporal association between exposure to GBCAs in patients with ESRD and the development of NSF [9].
These publications were of particular interest given the history of GBCA sales in the United States. In 1996, shortly before the identification of NSF, a paper declaring high-dose GBCAs to be significantly less nephrotoxic than iodinated contrast agents had been published [10, 11]. This observation implied that GBCAs were safe for use in renally compromised individuals, prompting the nearly reflexive use of these agents in patients with any degree of renal insufficiency in the years to follow [10, 11]. Thus, just as GBCAs were being preferentially employed for imaging studies in patients with kidney disease, the initial publications linking GBCAs and NSF began to appear. Ironically, GBCAs remained the agent of choice in many centers even in anuric ESRD patients who had no residual renal function to protect.
In June 2006, the U.S. Food and Drug Administration (FDA) issued its first advisory on the use of GBCAs in patients with kidney failure [12]. The public health advisory suggested that GBCAs should only be used if absolutely necessary in patients with advanced kidney failure, as defined by dialysis dependence or patients with a glomerular filtration rate (GFR) of 15 cc/min or less [12]. Additionally, the warning suggested that physicians should consider prompt initiation of dialysis following gadolinium contrast MRAs in patients with kidney dysfunction [12].
In 2007, two articles linking gadolinium to the onset of NSF strengthened the epidemiological data [13, 14]. The first article used a field emission scanning electron microscope equipped with energy dispersive spectroscopy to determine that the element, gadolinium (Gd) was detectable within the tissue of patients (Fig. 12.3) with NSF up to 11 months following exposure to GBCAs [13]. The second study used inductively coupled plasma mass spectrometry to illustrate that Gd levels were quantifiable within the tissue of patients with NSF [14]. Importantly, Gd levels were found to be highest in the tissue regions most affected by fibrosis [14]. Up until that time, pharmaceutical companies had assumed that no residual Gd would be retained in the body so long after the exposure.


Fig. 12.3
Field emission scanning photomicrograph showing aggregated particles of gadolinium associated with a cell body of a probable macrophage located within dermal fibrosis (Original magnification: ×32,000). (Reprinted from Journal of the American Academy of Dermatology, Vol 56(1), High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis, Pages 21-6, 2007, with permission from Elsevier and the American Academy of Dermatology)
That same year, the European Medicines Agency (EMEA) reviewed the safety profile for GBCAs in patients with kidney dysfunction, ultimately contraindicating the use of gadodiamide, gadopentetate dimeglumine, and gadoversetamide in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 in February, June, and July of 2007, respectively [10]. In 2010, the FDA followed suit, requiring all GBCAs to be labeled with warnings surrounding the risk of developing NSF for patients with acute kidney injury (AKI) or CKD stages 4–5 exposed to gadolinium, taking the additional step of specifically contraindicating gadodiamide, gadopentetate dimeglumine, and gadoversetamide use in patients with kidney disease [15]. The FDA also advised health-care professionals to evaluate kidney function in patients at risk of having kidney disease prior to the administration of GBCAs; to avoid using GBCAs in patients at risk for kidney failure; to monitor patients for signs and symptoms of NSF following GBCA administration; and to limit the dose of GBCAs used for imaging purposes [15].
Prior to 2007, sales figures for gadodiamide, the most commonly used GBCA, were on the rise [16]. However, following the publication of these regulatory guidelines, a reduction in overall sales of gadodiamide occurred [16]. In addition, as use patterns and protocols for GBCA administration changed following the publication of international guidelines, NSF incidence rates decreased significantly (Fig. 12.4), with a virtual disappearance of new cases reported to the registry by 2010 [16–20].
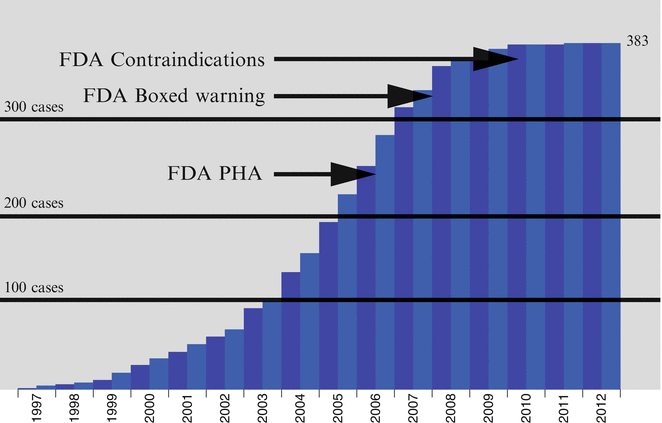

Fig. 12.4
Chart showing cumulative NSF cases reported to the NSF Registry by date of onset through 2012. Important FDA announcements are marked on the timeline. (With permission of the International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR.org)). FDA Food and Drug Administration, PHA Public Health Advisory
Physiology/Pathogenesis
Gadolinium Based Contrast Agents
The development of NSF requires: (1) GBCA exposure in the setting of (2) reduced renal function. In its ionic form, Gd3+ is a known toxin due to its ability to block voltage-gated calcium channels [21]. The high number of unpaired electrons in Gd3+ has the advantage of modifying proton relaxation time, a parameter that made it an ideal magnetic resonance imaging (MRI) contrast agent, providing the toxicity could be avoided [21]. Binding the Gd3+ to an organic ligand (chelate) seemed to enable the safe use of Gd in this context [9, 22]. Chelated gadolinium was initially believed to be safe for use in all patients, even those with kidney disease, and provided an alternative to nephrotoxic iodine-based contrast agents [11]. The tight bond between Gd and its ligand, and the relatively short half-life of GBCAs in healthy individuals, made these agents safe for use in imaging studies [9]. As a result, the FDA approved GBCAs for use as a contrast agent in MRI studies. However, because GBCAs were considered to have such a high safety profile, these agents were also routinely employed in MRA, an off-label use that required dosing at 2–3 times the FDA approved dosage for routine MRI examinations [23].
Nine GBCAs are currently available (Fig. 12.5), all of which are approved for use in the United States (Table 12.1) [24]. Following the initial publications linking gadolinium exposure to NSF and subsequent observations that suggested breakdown of the gadolinium-chelate complex (dechelation) might be partly to blame, additional studies began to focus on prolonged GBCA stability [9, 10, 24–26]. The risk for developing NSF is highest among patients who had received one of the three high-risk linear GBCAs, with essentially no or extremely rare verified cases of NSF following the use of the more stable macrocyclic agents, implicating a key role for stability of GBCAs in the pathogenesis of NSF [24].
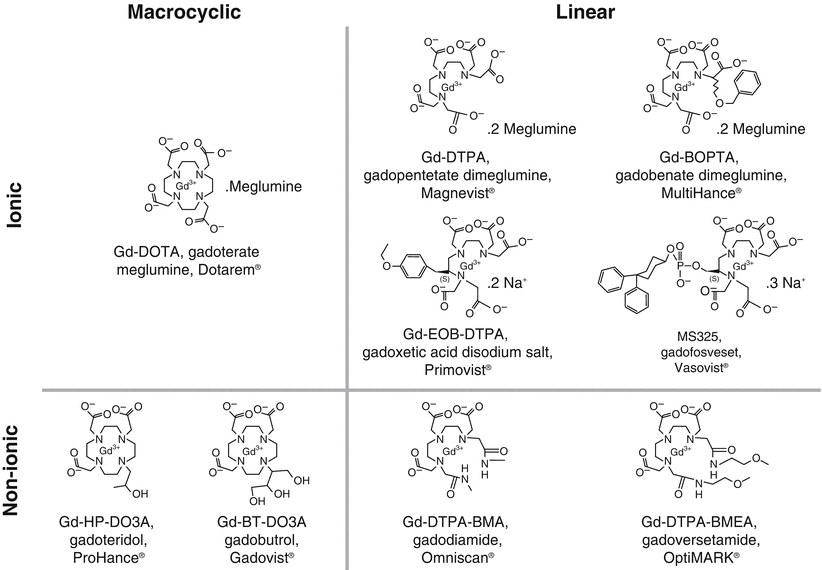

Fig. 12.5
Chemical structures of available GBCAs. (Reprinted from Radiologic clinics of North America, Vol 47(5), Idée JM, Port M, Dencausse A, Lancelot E, Corot C. Involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: an update, Pages 855-69, 2009, with permission from Elsevier)
Table 12.1
Classification of GBCAs by structure and charge
Generic name | Trade name | Structure/charge |
|---|---|---|
Gadodiamide | Omniscan™ | Linear nonionic |
Gadoversetamide | OptiMARK™ | Linear nonionic |
Gadofosveset trisodium | Ablavar®/Vasovist® | Linear ionic |
Gadoxetic acid disodium | Eovist®/Primovist® | Linear ionic |
Gadopentetate dimeglumine | Magnevist® | Linear ionic |
Gadobenate dimeglumine | MultiHance® | Linear ionic |
Gadobutrol | Gadovist®/Gadavist® | Macrocyclic nonionic |
Gadoteridol | ProHance® | Macrocyclic nonionic |
Gadoterate meglumine | Dotarem® | Macrocyclic ionic |
Currently available GBCAs enclose a single Gd atom within a proprietary organic ligand in either a linear or macrocyclic arrangement. Macrocyclic chelates enclose the Gd atom within a carbon ring. This arrangement confers stability, as multiple bonds must be broken simultaneously in order to release ionic gadolinium. Conversely, linear chelates wrap around the gadolinium atom, and can be sequentially unzipped from Gd by breaking single bonds. Both linear and macrocyclic chelates can also be either “ionic” or “non-ionic,” the latter generally regarded as being less stable [24].
Three GBCAs in particular have been implicated in the development of NSF: Gadodiamide (Omniscan™—GE Healthcare Inc., Princeton, NJ), gadopentetate dimeglumine (Magnevist®—Bayer Healthcare, Wayne, NJ), and gadoversetamide (OptiMARK™—Mallinckrodt, St. Louis, MO) [10, 24]. Of these three agents, two (gadodiamide and gadoversetamide) utilize a linear non-ionic chelate, and one (gadopentetate dimeglumine) utilizes a linear ionic chelate [10].
Almost all GBCAs are cleared through renal dependent mechanisms and reduced GFR is a known prerequisite for the development of NSF [27]. In healthy subjects, the elimination half-life for most GBCAs is 1.3 h ± 0.25 h. In patients with reduced kidney function, the half-life of GBCAs was found to be prolonged up to 34.3 h ± 22.9 h in CKD stage 4, suggesting that reduced renal function results in an extended exposure to GBCA during which an adverse reaction might occur [28]. In patients with ESRD, GBCA will only be cleared when dialysis is performed, and it can take up to four hemodialysis sessions for removal of one GBCA dose [28].
Although a majority of patients have developed NSF in the setting of ESRD, approximately 20 % of patients experience disease onset in the setting of AKI or CKD stages 4 or 5 [24]. A model for calculating risk in patients (Fig. 12.6) with varying levels of CKD has been developed, based upon an estimated risk of 2.4 % for developing NSF in ESRD patients exposed to a high risk GBCA. Utilizing estimated prevalence for CKD in the US and distribution of NSF among patients with various levels of kidney function based on reports in the literature, the model predicts that patients with CKD 4 have a 1 in 2,492 risk of developing NSF after exposure to GBCA. The risk for patients with CKD stage 3 drops to between 1 in 100,000 and 1 in 700,000, suggesting that a patient with CKD stage 3 who is exposed to a single dose of a stable GBCA is not at an increased risk for developing NSF [18, 24]. For patients with ESRD, peritoneal dialysis-dependent patients were found to be at a 7.5 % increased risk of developing NSF as compared to hemodialysis-dependent patients [6]. Finally, only 2–18 % of patients with reduced renal function who are exposed to GBCAs ultimately develop NSF, suggesting that additional factors contribute to the pathogenesis of this disease [24, 26, 29–33].
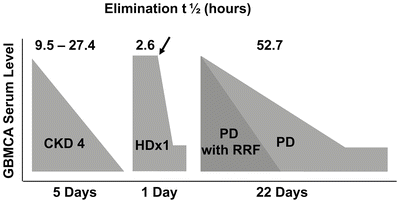

Fig. 12.6
Schematic representation of prolonged elimination of gadolinium-based magnetic resonance contrast agents in chronic kidney disease (CKD), hemodialysis (HD), and peritoneal dialysis (PD), with or without residual renal function (RRF). Arrow indicates initiation of hemodialysis. (Reprinted from Journal of the American College of Radiology, Vol 5(1), Abu-Alfa A. The impact of NSF on the care of patients with kidney disease, Pages 45-52, 2008, with permission from Elsevier and the American College of Radiology)
Circulating Fibrocytes
In 2003, circulating fibrocytes (CF) were identified as the likely effector cells in NSF [34]. CFs were first described in 1994 as a subpopulation of circulating leukocytes (Fig. 12.7) with the ability to differentiate into fibroblast-like cells to facilitate wound repair at the local level [35]. However, in addition to normal wound repair, CFs have also been implicated in the propagation of several fibrosing conditions [36]. In 2003, the observation that the spindle-shaped cells seen in biopsies from patients with NSF stained for both CD34 and procollagen I (Fig. 12.8) led to the hypothesis that aberrant CF activity was to blame [34, 36]. Why this population of cells was emerging from the circulation and homing to the particular sites of fibrosis in NSF was unclear [34].
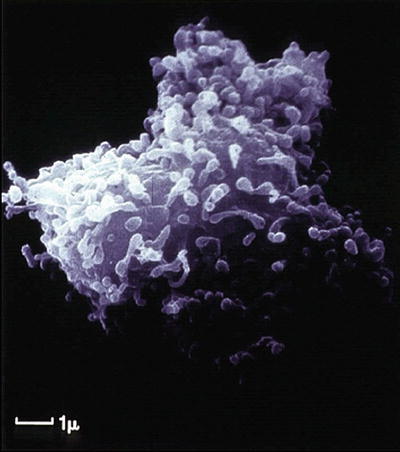
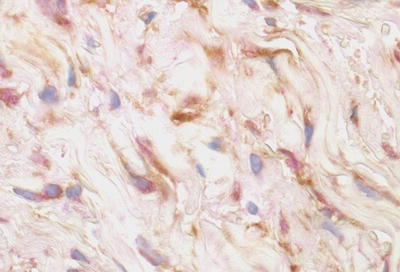

Fig. 12.7
Scanning electron micrograph of a human circulating fibrocyte. (Reprinted from the International Journal of Biochemistry & Cell Biology, Vol 36(4), Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood, Pages 598-606, 2004, with permission from Elsevier)

Fig. 12.8
Double immunoperoxidase staining for CD34 (brown) and procollagen (red) shows staining for CD34 is most marked along the dendritic processes while that for procollagen is localized to the cytoplasm. (From Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. American Journal of Dermatopathology 25(4), Page 358, 2003. Reprinted with permission)
Shortly after the epidemiologic link between GBCAs and NSF was identified, a cell culture study by Edward et al. explored some of the key differences between NSF and control fibroblasts as well as the effects of gadolinium on fibroblast function [37]. The study showed that fibroblasts from patients with NSF demonstrated higher levels of hyaluronan and collagen production than those from controls. Additionally, control fibroblasts demonstrated increased production of these matrix materials after exposure to the serum of patients with NSF. Finally, when control fibroblasts were exposed to gadodiamide itself, they were shown to have increased growth, matrix production, and an enhanced ability to differentiate into myofibroblasts [37]. Additional studies confirmed the stimulatory role of gadolinium on the proliferation of fibroblasts [38] and the in vitro pro-fibrotic activity of the gadolinium-chelate complex [39].
Animal Studies
To date, most of the animal studies of NSF were planned and executed by contrast manufacturers. Until recently, these depended on a sub-totally nephrectomized rat model [22, 40–43]. However, this model did not fully capture the clinical picture present in NSF. A subtotal nephrectomy leaves the animal with a diminished GFR (as compared to an absent GFR in ESRD patients) and is not accompanied by the same degree of hyperphosphatemia typically observed in ESRD patients [22]. Nevertheless, these models were collectively able to demonstrate that the GBCA gadodiamide was capable of producing histopathological changes in the 5/6 nephrectomized animals similar to those found in human patients with NSF (Fig. 12.9) [44]. Additionally, deposition of gadolinium in the skin of these animals was measured and found to be highest for animals dosed with gadodiamide and other linear agents, when compared to levels found in animals exposed to macrocyclic agents [22].
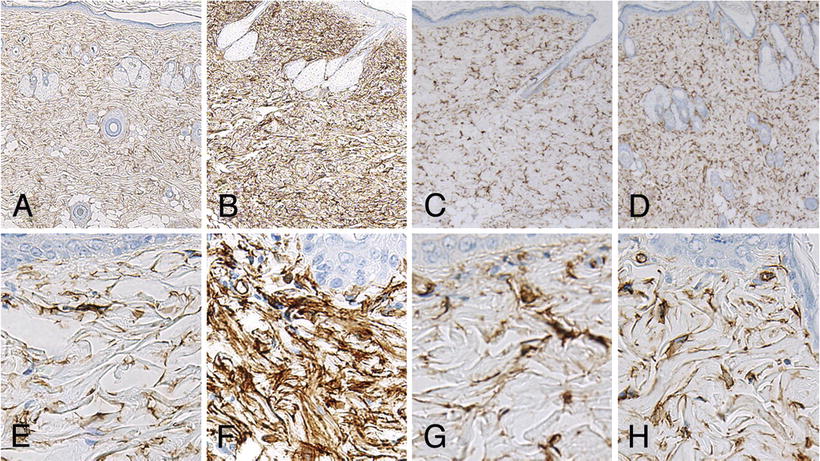

Fig. 12.9
Microscopic skin findings in rats after final administration of each agent. High-magnification images show strong diffuse CD34-positivity among spindle cells of the dermis in the gadodiamide group (b, f). Column 1: control, Column 2: gadodiamide, Column 3: gadoxetate sodium, Column 4: Gadoteridol. (Images a–d ×50 magnification, Images e–h ×400 magnification). (Reprinted from Magnetic Resonance Imaging, Vol 31(8), Sato T, Ito K, Tamada T, Kanki A, Watanabe S, Nishimura H, Tanimoto D, Higashi H, Yamamoto A. Tissue gadolinium deposition in renally impaired rats exposed to different gadolinium-based MRI contrast agents: evaluation with inductively coupled plasma mass spectrometry (ICP-MS), Pages 1412-7, 2013, with permission from Elsevier)
More recently, Fretellier et al. have been able to achieve a more accurate rat model replication of NSF pathology [22]. This group’s model utilized rats on an adenine-enriched diet for 8, 14, or 16 days to produce differing degrees of renal failure within the context of hyperphosphatemia. Rats with differing degrees of renal failure were then exposed to either gadodiamide or saline. Serial biopsies were obtained to allow for histologic examination and quantification of gadolinium within the tissue using inductively coupled plasma mass spectrometry. This trial demonstrated that rats with more severe kidney dysfunction had more severe profibrotic skin lesions and systemic toxicity. Additionally, rats that developed the most severe pathology were also found to have higher gadolinium levels by mass spectrometry than rats without cutaneous changes. Akin to humans, affected rats demonstrated overexpression of profibrotic cytokines including transforming growth factor beta-1 (TGF-β1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) [22].
Another trial by this group examined the effects of exposing the rats with the greatest degree of kidney dysfunction to each of the available GBCAs. As opposed to the gadodiamide trial with differing degrees of renal compromise, these rats did not produce cutaneous changes, likely due to the high degree of toxicity and early mortality in this trial [22]. However, the authors were able to perform relaxometry studies that revealed that both linear GBCAs (gadodiamide and gadopentetate) underwent slow, gradual dechelation (defined by a release of the gadolinium ion from its organic chelate) in the adenine-induced renal failure rats. Conversely, all macrocyclic agents remained stable and did not produce a measurable degree of dechelation in vivo. To date, this model has most closely mimicked the pathologic process of NSF in vivo and provided additional insight into possible mechanisms at play behind the role of gadolinium in NSF [22].
Clinical
In 2003, 6 years of demographic data were analyzed from Yale’s International NSF registry to develop an epidemiologic profile for this disease [4]. The age range of NSF patients at disease onset is 8–87 years, with a mean age of 46.4 years [4]. No gender or racial predilections have been observed [4]. The majority of patients diagnosed with NSF have ESRD at the time of diagnosis [24]. However, approximately 20 % of patients develop NSF with CKD stages 4 or 5 or AKI (Fig. 12.10) [24]. Additionally, patients with NSF have an increased likelihood of: (1) hypercoagulability leading to clotting complications; (2) vascular surgical procedures in the weeks to months prior to NSF diagnosis; (3) and brain tumors. The currently accepted interpretation of these observations is that each is associated with magnetic resonance (MR) imaging or angiography that eventually leads to a GBCA exposure.
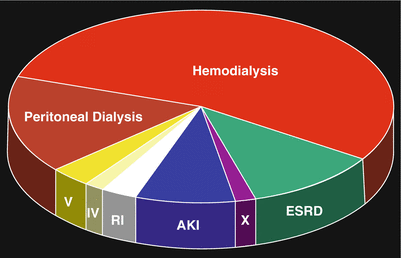

Fig. 12.10
Renal status of patients upon onset of NSF. Hemodialysis (52 %); peritoneal dialysis (16 %); end-stage renal disease (11 %); acute kidney injury (AKI) (10 %); renal insufficiency/CKD stage unknown (RI) (8 %); CKD stage IV (1.5 %); CKD stage V (3 %); post-transplant (X) (3 %). (With permission of the International Center for Nephrogenic Systemic Fibrosis Research (ICNSFR.org)). ESRD end-stage renal disease, AKI acute kidney injury
The earliest observable clinical effects of NSF include erythema, edema, and palpable warmth of the affected extremity, often leading clinicians to suspect cellulitis. Patients with NSF also commonly describe pain or pruritus in affected areas, and over time, joint complaints that include pain and stiffening. Almost all patients develop indurated plaques with a woody consistency (Fig. 12.11). These have a predilection for the distal extremities, with the legs more commonly involved than the arms. Induration and fibrosis adjacent to joints can become severe enough to produce irreversible contractures (Fig. 12.12). Many patients have deteriorated from being fully ambulatory to wheelchair-restricted within a few weeks to months. Other common findings include “cobblestoning,” where the skin develops raised papules over an area of induration visually resembling a cobblestoned sidewalk, and “peau d’orange,” wherein surface changes overlying deep cutaneous tethering come to resemble the texture of an orange peel [4, 27].
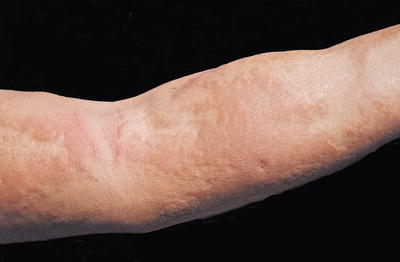
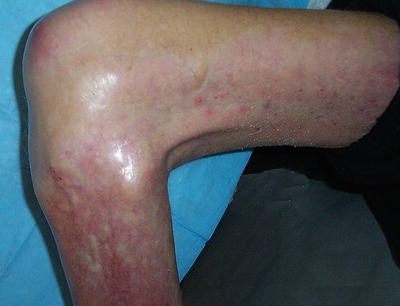

Fig. 12.11
The forearm of a patient manifesting the thickened, brawny plaques of NSF. The surface can appear “cobblestoned” or have a “peau d’ orange” texture. (From Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. American Journal of Dermatopathology 23(5), Page 383-93, 2001. Reprinted with permission)

Fig. 12.12
In “end-stage NSF,” the skin becomes fibrotic, and subcutaneous tissue is diminished. Contracture of the knee is illustrated. (Reprinted from Journal of the American College of Radiology, Vol 5(1), Girardi M. Nephrogenic systemic fibrosis: a dermatologist’s perspective, Pages 40-4, 2008, with permission from Elsevier and the American College of Radiology)
Cutaneous manifestations can also include puckering or linear banding, superficial plaques and/or patches, and dermal papules. Cutaneous manifestations only rarely involve the trunk or face [1, 4, 27]. Scleral plaques have been described in a number of patients with NSF (Fig. 12.13) [45]. Because patients with NSF can present with a constellation of symptoms, a diagnostic system combining major and minor clinical criteria has been established [27]. Clinical evaluation, however, is not sufficient to fully exclude competing clinical differentials (Table 12.2). All patients who present with clinical findings suggestive of NSF should undergo an incisional or deep punch biopsy of involved skin to obtain a specific diagnosis. A combination of clinical (Tables 12.3 and 12.4) and histopathological features (Table 12.5) informed by concurrent historical and laboratory evaluation (Table 12.6), should accurately identify most putative cases of NSF.
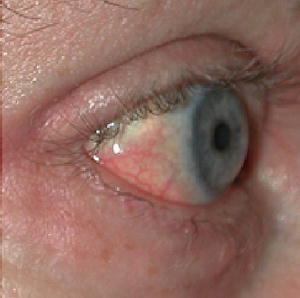

Fig. 12.13
Scleral plaques (minor criterion). There are new onset white-yellow scleral plaques with dilated capillary loops in a patient age <45 years. Over this age, the sign becomes less specific due to overlapping features with actinically induced pingueculae. (Reprinted from Journal of the American Academy of Dermatology, Vol 65(6), Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations, Pages 1095-1106, 2011, with permission from Elsevier and the American Academy of Dermatology)
Table 12.2
NSF: clinical and histopathological differential diagnoses
Clinical differential diagnoses | Histopathological differential diagnoses |
|---|---|
Lipodermatosclerosis/chronic venous stasis | Lipodermatosclerosis/chronic venous stasis |
Scleromyxedema | Scleromyxedema |
Eosinophilic fasciitis | Eosinophilic fasciitis |
Scleroderma (systemic sclerosis) | Morphea/scleroderma |
Scleredema diabeticorum | Scleredema diabeticorum |
Porphyria cutanea tarda (sclerodermoid) | Eosinophilia-myalgia syndrome |
Morphea/Lichen sclerosus et atrophicus | Lipodermatosclerosis |
Chronic graft-versus-host disease | Chronic graft-versus-host disease |
Stiff skin syndrome/congenital fascial dystrophy | Stiff skin syndrome/congenital fascial dystrophy |
Diabetic digital sclerosis | Septal panniculitis |
Pruritus of renal disease/neuropathy | Pseudoxanthoma elasticum |
β2-microglobulin amyloidosis | Calciphylaxis |
Dupuytren contracture | Dermatofibrosarcoma protuberans |
Table 12.3
Clinical criteria, scoring summary
Major criteria | Minor criteria |
|---|---|
Patterned plaques | Puckering/linear banding |
Joint contractures | Superficial plaque/patch |
“Cobblestoning” | Dermal papules |
Marked induration/“Peau d’orange” |









