Promotion of moist wound healing4
 Reduction of edema and interstitial fluid 1,4,5
Reduction of edema and interstitial fluid 1,4,5
 Approximation of wound edges4,9
Approximation of wound edges4,9
 Stimulation of granulation tissue formation9,10
Stimulation of granulation tissue formation9,10
 Reduction in bacterial load9
Reduction in bacterial load9
 Reduction in the frequency of dressing changes11
Reduction in the frequency of dressing changes11
Since its acceptance into mainstream wound management, NPWT has been utilized in treating a wide variety of acute and chronic wounds12 including acute traumatic and surgical2,13 wounds healing by primary and secondary intention; burns14,15; and chronic wounds associated with venous insufficiency,3,12 diabetes,3,10,16,17 and pressure.18 Events such as the devastating 2010 earthquake in Haiti14 and war in the Middle East14,19,20 have also shown how the benefits of NPWT can positively influence limb salvage rates and decrease morbidity and mortality in mass casualty and high-energy injury situations.
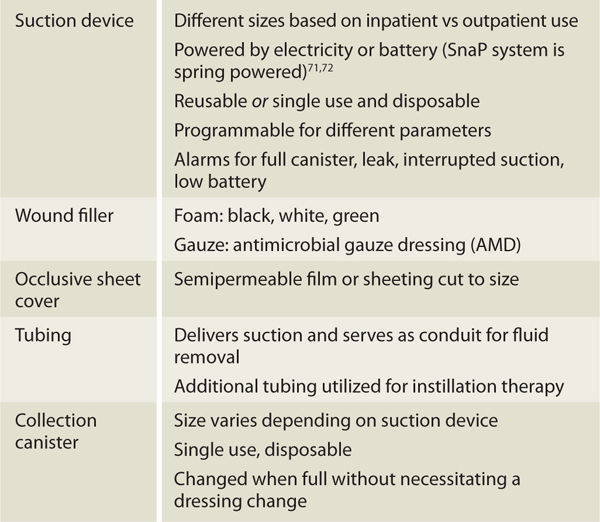
NPWT systems consist of a pump unit that provides suction, a wound filler that transfers negative pressure to the wound bed and allows flow of fluids from the wound, a transparent occlusive sheet that covers the wound filler and creates an airtight seal, and flexible tubing that delivers suction and serves as a conduit for removal of drainage and wound debris.21 Wound fluids are collected in a disposable container that is attached to the pump (FIGURE 15-1). TABLE 15-1 further describes NPWT components.
FIGURE 15–1 Negative Pressure Wound Therapy The fluids are suctioned from the wound, through the foam, into an attached tube and into an air tight disposable canister. Although every system has its own unique characteristics, the basic functions of removing interstitial edema, stimulation cell proliferation, and reducing wound size are the same.
TABLE 15-1 Basic NPWT Components
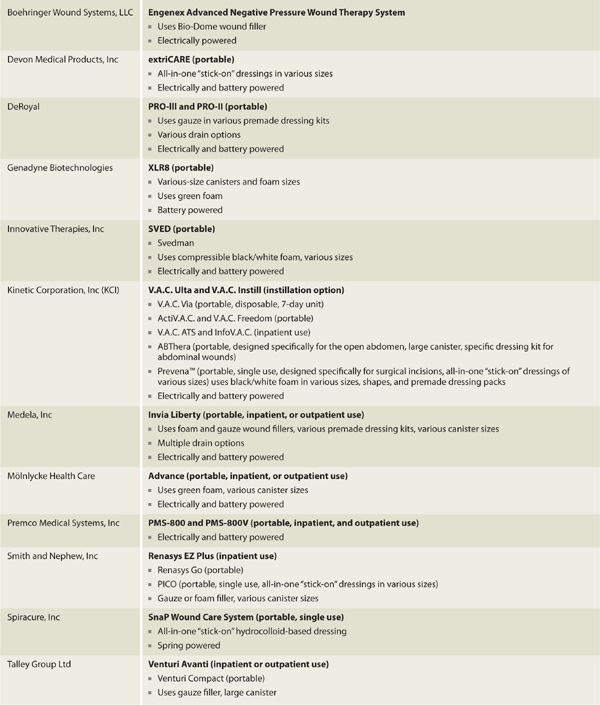
The first commercially available NPWT device marketed in the United States as a wound healing device22 was the Vacuum Assisted Closure or V.A.C. (Kinetic Concepts, Inc (KCI), San Antonio, TX). The initial V.A.C. device was a somewhat bulky, electrically powered unit designed primarily for inpatient use.22 A smaller, portable, battery-operated unit was designed a short time later for use in the outpatient setting.3,15 Over the past few years, an explosion in NPWT interest and development has occurred and now multiple vendors offer relatively silent NPWT devices in various sizes that are reusable, recyclable, or disposable with some units being specially designed for specific types of wounds (see TABLE 15-2 for partial vendor list). Increased healing, portability, and ease of management can result in decreased length of hospital stay, faster return to function,23 cost savings,24 and improved quality of life.
TABLE 15-2 Partial NPWT Vendor List
THEORY
NPWT promotes wound healing primarily through removing wound fluid, mechanical stimulation of cells, and occlusion of the wound from environmental contaminants. As fluid is suctioned from the wound, interstitial edema is reduced. Inflammatory mediators and bacteria are removed along with tissue fluids, thereby reducing wound inflammation and facilitating progression to the proliferative healing phase. Mechanical stimulation at both the tissue (macrostrain) and cellular (microstrain) levels25 encourages cellular proliferation, granulation tissue formation, and wound contraction.13,26 The occlusive covering maintains a moist wound healing environment and an airtight seal that is vital in sustaining negative pressure.1,5,13,27 Despite differences in size and shape, all NPWT devices basically offer the same benefits, and selection of a device may depend on the patient’s medical coverage. Most devices offer several options for “tailoring”21 NPWT to each patient need so that optimal care can usually be achieved regardless of specific vendor.
EFFECTS AT THE CELLULAR AND TISSUE LEVELS
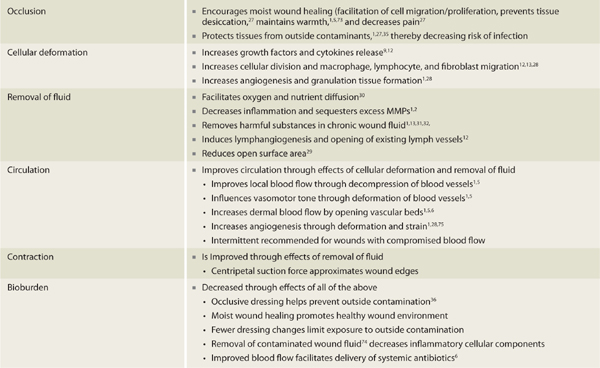
NPWT has several documented effects at the cellular and tissue levels. Due to the close, interrelated nature of these effects, cellular and tissue changes associated with NPWT are discussed together and presented in the following main categories: occlusion, cellular deformation, fluid removal, circulation, contraction, and bioburden (TABLE 15-3).
TABLE 15-3 Summary of NPWT Cellular and Tissue Effects
Occlusion
The benefits of moist wound healing have been well established.1,5,13,27 Since NPWT requires an airtight seal in order to maintain suction, all devices require that an occlusive dressing be applied over the open wound site. As long as the seal is maintained, a moist wound environment is created. NPWT dressings are changed every 48 to 72 hours; thus, exposure of the wound to air (which can decrease wound bed moisture and temperature, and subsequently slow down the healing process) is reduced and the moist wound environment preserved.
Cellular Deformation
The suction force associated with NPWT causes mechanical deformation of cells.2,13,28 Referred to as microstrain12,14,25 or simply strain,28 the stretching force creates changes within the cells that result in increased release of growth factors and cytokines associated with cellular proliferation.9,12 Increased cellular division and migration of macrophages, lymphocytes, and fibroblasts are also facilitated.12,13,28 Mechanical deformation caused by negative pressure results in increased angiogenesis and granulation tissue formation and is one of the most significant effects of NPWT.1,28 DeFranzo et al29 demonstrated that in healthy, noninfected wounds, newly formed granulation tissue is routinely present within 48 hours after NPWT is initiated.
Fluid Removal
The removal of interstitial wound fluid is a significant factor associated with NPWT.1,5,13 Removal of edema associated with acute injury and trauma facilitates oxygen and nutrient diffusion30 as well as decreased inflammation as inflammatory mediators are removed with wound fluid.1,2 It has been well documented that chronic wound fluid is detrimental to wound healing1,31–33 and due to its unique method of fluid removal, NPWT is especially beneficial in the treatment of chronic wounds.29 Studies conducted by Labanaris34 and Dini et al12 show that NPWT induces proliferation of lymph vessels and suggest that this aides in quick reduction of wound fluid especially in the first 4 days of treatment. Dini et al12 also demonstrated that NPWT encouraged opening of collapsed lymph vessel lumens in chronic venous insufficiency wounds. In many cases, tissue edema can be significantly resolved within 3 to 5 days, thereby facilitating reduction of wound surface area fairly quickly.29
Circulation
Local circulation is improved with NPWT6,7,8,13,25,28 through the combined benefits of cellular deformation and fluid removal. In an animal study, Borgquist8 demonstrated improved blood flow at 1.0 and 2.5 cm from the wound edge with the application of negative pressure. Conversely, it has been shown that due to compression of tissues, blood flow is actually decreased at the superficial wound edge and may extend as far as 0.5 cm from the wound edge indicating that NPWT may create a gradient of blood flow changes.6,7,30 Researchers believe hypoperfusion at the wound edge acts as a stimulus for angiogenesis and is therefore seen as a beneficial effect of NPWT.6,30 NPWT-induced hypoperfusion may not, however, be beneficial in situations where blood flow is already significantly compromised; further decreases in blood flow could result in ischemia and tissue necrosis. In this case, the use of lower negative pressures would be appropriate so that compression of tissue at the wound edge is reduced.8 Also, when wound edge blood flow is a concern, intermittent NPWT may be more appropriate than continuous therapy since the on/off cycles provide decompression or rest periods that may decrease the risk of ischemia7(Intermittent and continuous therapy are discussed in more detail in the section on Parameters.) In all cases, since the amount of wound edge compression is pressure dependent, it is recommended that the lowest effective pressure be used in order to decrease potential complications with hypoperfusion.21
Contraction
Studies support that the centripetal1 pulling or suction force of NPWT assists in approximating wound edges and with wound contraction.13,35 This action, referred to as “macrostrain,”9,14,25 combined with reductions in localized tissue edema via fluid removal, effectively reduces wound surface area. Additionally, wound edge macrostrain has been shown to mechanically stimulate larger cytoskeleton structures within granulation tissue, resulting in increased cellular proliferation.1,13,25
Bioburden
The exact method of how NPWT affects bacterial load remains unclear.9,14,19,21,35 A study conducted by Weed et al36 showed wound closure with NPWT even in the presence of 106 bacteria. Other studies have demonstrated improved healing though bacteria levels actually increased during NPWT use.35 It appears that NPWT positively affects wound bioburden through all of the mechanisms discussed above.37 Some of the newer NPWT units provide for the instillation of topical solutions as another mechanism of combating bacteria. Overall, NPWT is just one component in a treatment strategy for highly contaminated or infected wounds13,16,28 and should be combined with other standard therapies, including sharp/surgical debridement, systemic antibiotics, irrigation with pulsed lavage with suction (see Chapter 17), and topical antibacterials.35 In 2009, a multidisciplinary expert panel stressed this point by identifying prerequisite recommendations regarding the use of NPWT on infected wounds. These recommendations include the following: the patient is free of systemic signs of infection, necrotic tissue is debrided and abscesses are drained, and perfusion is adequate for healing(TABLE 15-4 and FIGURES 15-3, 15-4).38
TABLE 15-4 Prerequisites to NPWT in Infected Wounds38
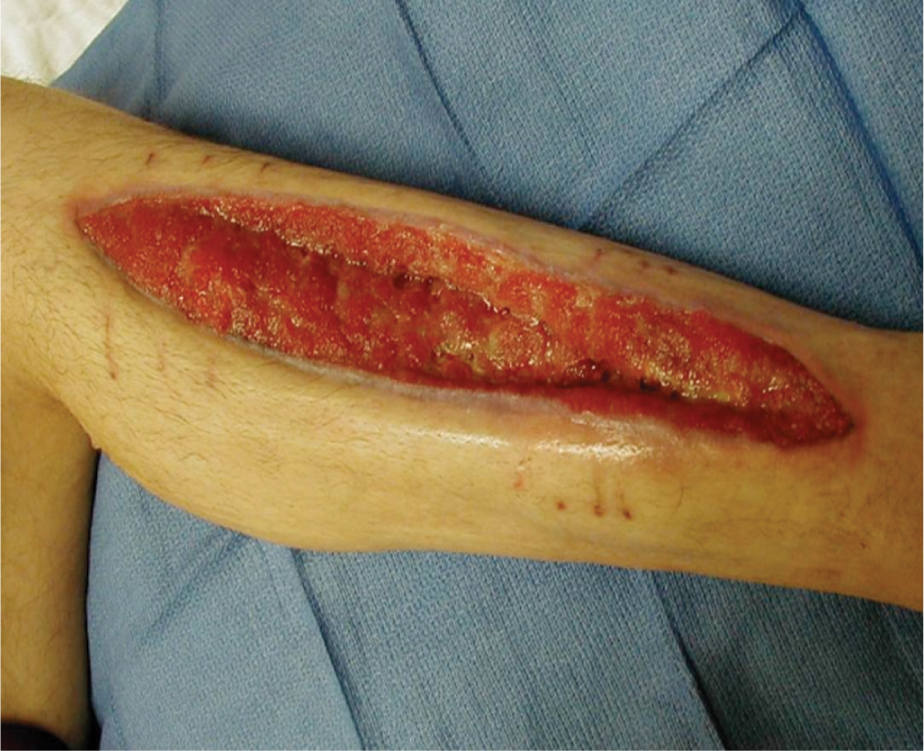
FIGURE 15–3 Wound not appropriate for NPWT This wound does not meet the criteria of having less than 30% of the wound bed devitalized tissue, and therefore would require more wound bed preparation before applying NPWT.
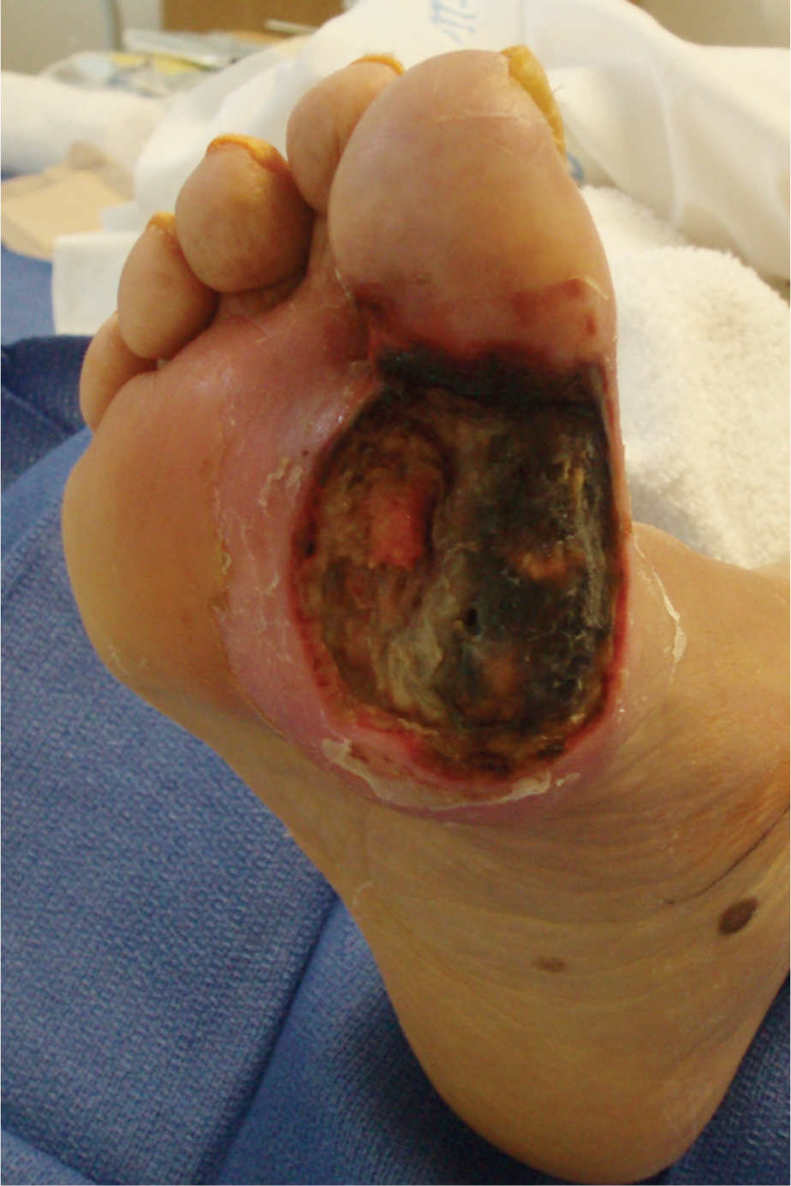
FIGURE 15–4 Wound not appropriate for NPWT Although this wound has been surgically debrided and is an appropriate shape for NPWT, the amount of necrotic tissue and lack of angiogenesis at the edges indicates that there is insufficient perfusion (or blood supply) for the wound to heal. Therefore, it is inappropriate for NPWT.
INDICATIONS
TABLE 15-5 provides a list of wound etiologies for which NPWT is indicated (FIGURES 15-5 to 15-12). The cellular and tissue effects need to be considered for each indication in order to obtain optimal patient outcomes and for future problem solving.
TABLE 15-5 Indications for NPWT

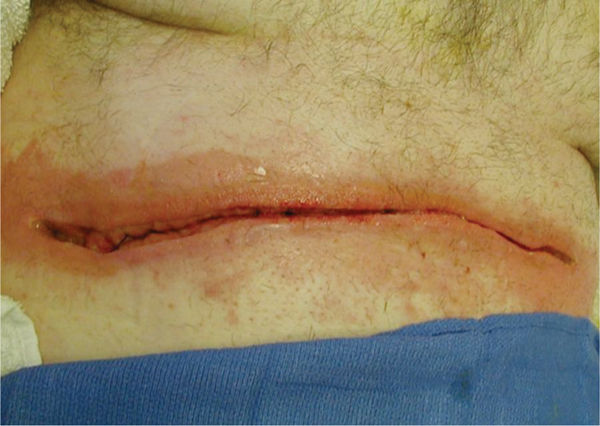
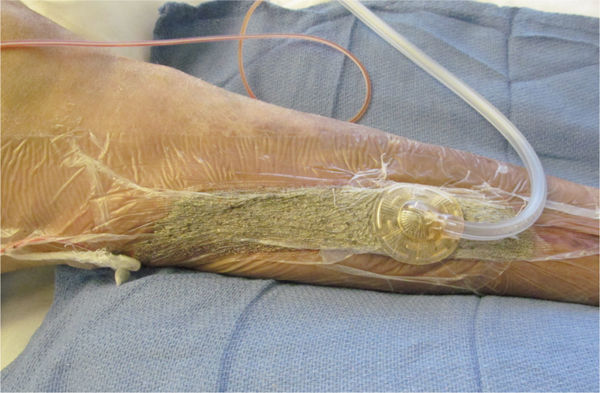
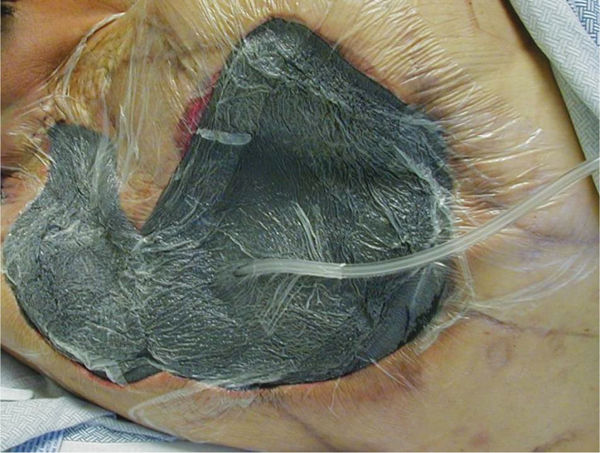
FIGURE 15–5 Abdominal incisional wound on a patient who has had a Whipple procedure for chronic pancreatitis The erythema on the periwound skin is a result of the copious drainage that has been coming from the wound. This wound is indicated for NPWT to promote granulation and closure, as well as to manage exudate and allow the skin to recover.
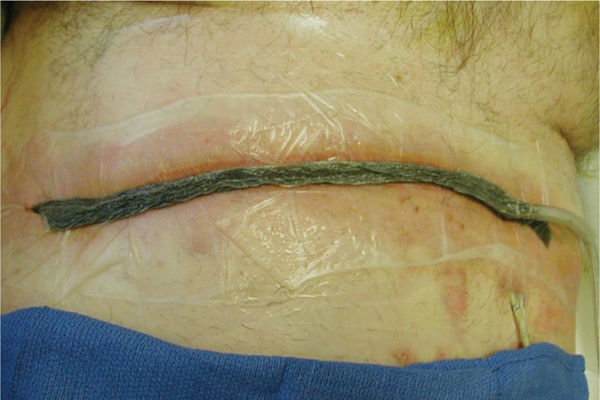
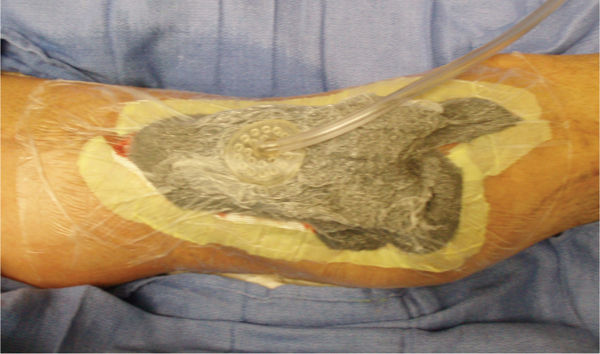
FIGURE 15–6 The abdominal wound with the NPWT in place Under the adhesive drape is a layer of Vaseline gauze to further protect the inflamed skin and to help minimize pain when the drape is removed. Also note the Chariker-Jeter or mesentery approach of applying the suction tube into the foam. This method is recommended for high-output wounds, small or narrow wounds where the round track pad may press on surrounding tissue, or gauze-filled wounds.
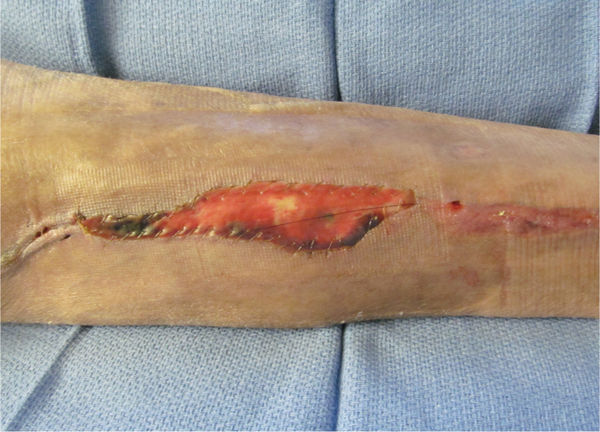
FIGURE 15–7 Wound over which a biological dressing has been attached with sutures The NPWT will bolster the dressing so that (1) it does not shear and distort new capillaries, (2) it will drain interstitial fluid, and (3) it will promote cellular mitosis.
FIGURE 15–8 Wound with NPWT applied using silver-impregnated foam Note how the drain at the proximal wound edge is incorporated into the dressing in order to ensure an adequate seal.
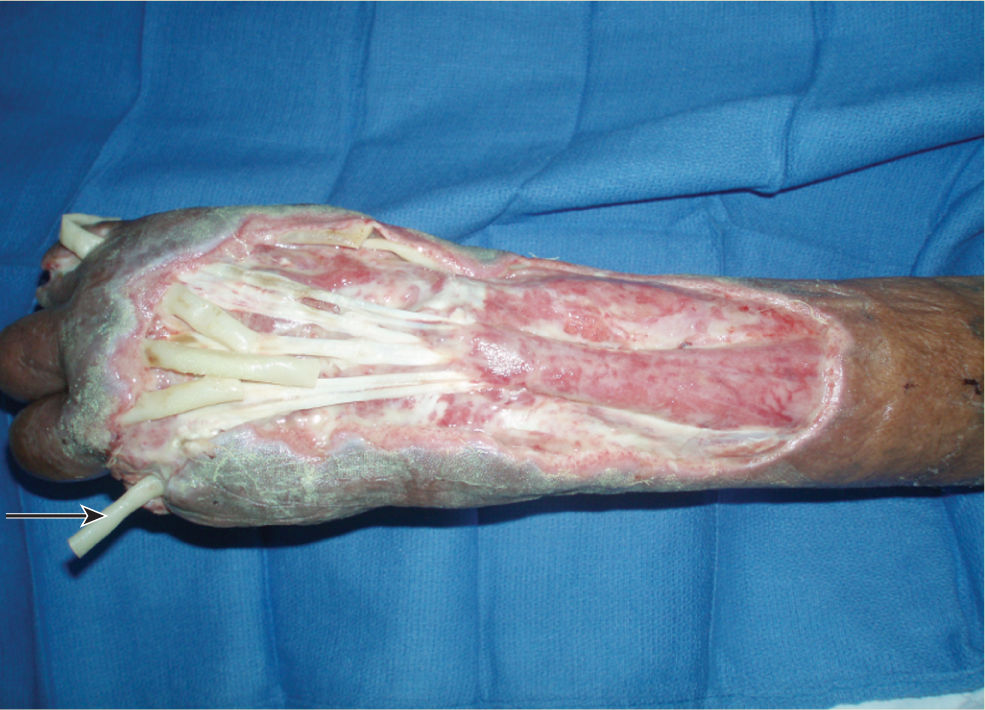
FIGURE 15–9 Lower extremity compartment syndrome after fasciotomy Treatment with NPWT has provided macrostrain that is facilitating wound contraction and microstrain that is facilitating angiogenesis and enhanced granulation.
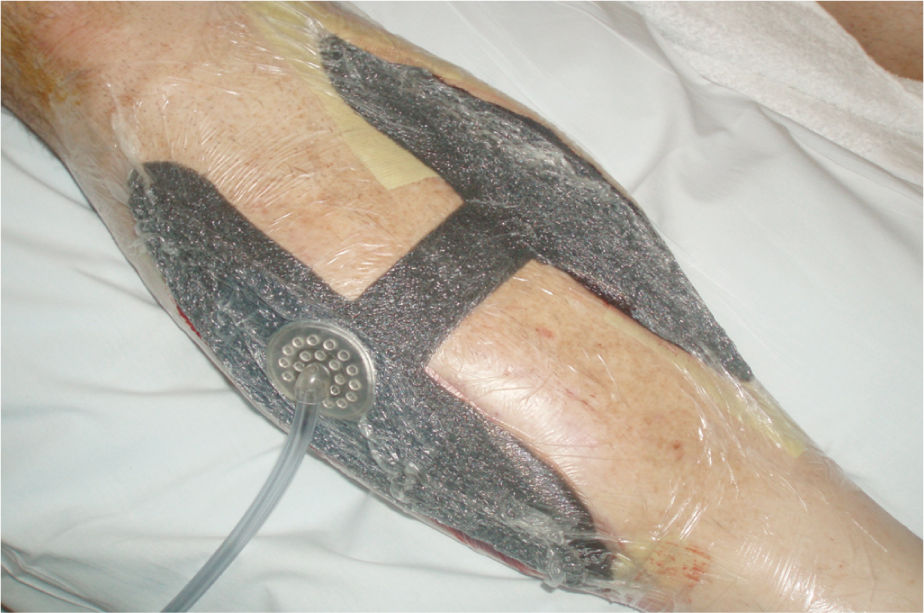
FIGURE 15–10 NPWT on medial and lateral lower extremity fasciotomies for compartment syndrome The foam bridge (with a layer of drape beneath to protect the skin) allows both wounds to be connected to one pump.
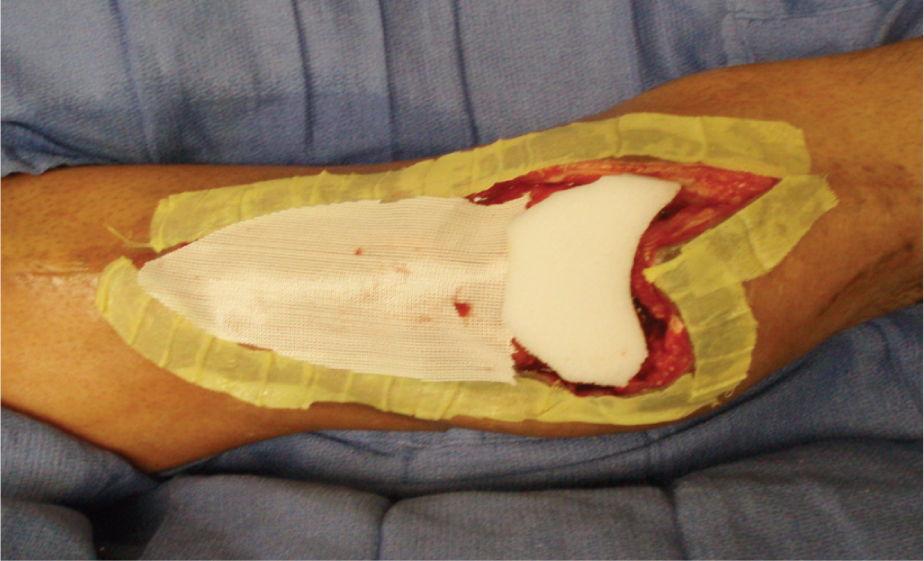
FIGURE 15–11 Traumatic wound on the lower extremity is prepared for NPWT application by placing white foam over expose bone, nonadhesive mesh over muscle, and Xeroform strips over the edges These strategies minimize both pain and damage to existing structures and new granulation tissue when the dressing is removed.
FIGURE 15–12 The wound in Figure 15-10 with the NPWT in place.
Acute Wounds
Evidence supports the use of NPWT for most acute surgical wounds13 and for traumatic2,29 wounds (especially those with exposed bone, tendon,1,24,39 or hardware1) that require increased granulation prior to surgical closure or grafting. One exception to acute surgical wounds is an abdominal wound with thin tissue protecting any part of the gastric system in which case the negative pressure may increase the risk of enterocutaneous fistula formation. Due to the cellular and tissue effects of NPWT, placement over a new split-thickness skin graft (STSG) has been shown to increase graft “take” through edema reduction and graft stabilization2,35 and is indeed considered by some as the standard of care for high-risk STSGs.2 When treatment is initiated and the wound filler compresses, NPWT can assist with stabilization or splinting of the wound site. DeFranzo and colleagues29 also found that NPWT utilized in complicated traumatic lower extremity, ankle, and foot injuries allowed patients to be more mobile sooner than with other dressings due to the increased stabilization. In mass casualty or battlefield situations, NPWT is used to stabilize and protect large open wounds until patients can be transported to advanced facilities for appropriate care.14,19,35
NPWT application over suture lines improves wound integrity by maintaining wound edge approximation and decreasing periwound tension, thereby decreasing the risk of dehiscence in high-risk patients.40–42 Disposable, 7-day NPWT units, such as Prevena (Prevena Incision Management System, KCI USA, San Antonio, TX) are available specifically for use with surgical incisions (FIGURE 15-13). NPWT may also be indicated for full-thickness burns after debridement of nonviable tissue has been performed.9,43 However, guidelines developed by an expert panel in 2010 recommended that the decision to use NPWT on full-thickness burns should only be made by a burn specialist (refer to Chapter 9 for more discussion about treatment of burns).w43 Application of NPWT to deep partial thickness burns may prevent progression of tissue damage due to improved circulation and edema reduction.43
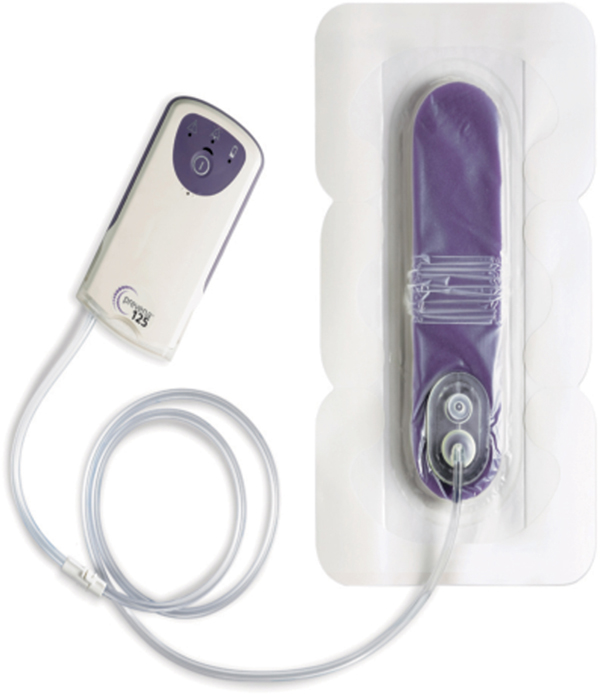
FIGURE 15–13 NPWT for surgical incisions The Prevena Incision Management System is a small NPWT unit that is placed over surgical incisions to help prevent dehiscence and surgical site infection. The disposable unit has a 45 mL canister and a peel-and-stick foam pad. It is applied over a clean surgical incision and left in place for the duration of the battery life. (PrevenaTM Incision Management System; Courtesy of KCI Licensing, Inc. 2013.)
Chronic Wounds
Successful use of NPWT has been documented for ulcers associated with pressure,1,29,44 venous insufficiency,13 and diabetes1 as well as chronic wounds related to reconstructive surgery26 and exposure to radiation.18 NPWT can also be effective in palliative care of terminal patients with highly exudating malignant wounds by reducing odor, controlling drainage, and decreasing dressing changes (FIGURE 15-14).1 NPWT in the presence of arterial insufficiency requires caution due to wound and periwound hypoperfusion as previously discussed.6,8,30.
FIGURE 15–14 Palliative care NPWT dressing on a large upper back wound where a malignant tumor was excised. The patient is on hospice, and the dressing is used to manage drainage, minimize pain, minimize dressing changes, and optimize quality of life for both patient and family members during end-of-life care.
Special Populations
NPWT has been used on patients of all ages including neonates and the elderly.23 Evidence documenting NPWT use on the very young stresses the need for special consideration regarding patient size and weight as neonates, infants, and children are more susceptible to dehydration.23 In pediatrics, pressure settings range from 50 to 125 mmHg and are dependent on age, wound etiology, and location.23 In 2009, Baharestani and expert colleagues23 published detailed best practice guidelines for the use of NPWT in pediatric patients (TABLES 15-6 to 15-8) and providers engaged in pediatric wound management are advised to read these guidelines. Dehydration can also be an issue for elderly patients and fluid levels should be monitored closely in patients with high-output wounds. Physicians should be notified if two collection canisters are filled within a 24-hour period.18
TABLE 15-6 NPWT Pressure Guidelines for Pediatric Patients23
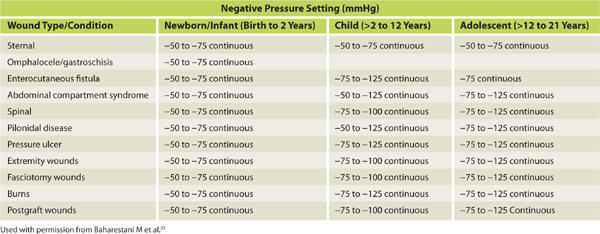
TABLE 15-7 NPWT Precautions for Pediatric Patients23
TABLE 15-8 NPWT Guidelines for NPWT Foam use for Pediatric Patients23
PRECAUTIONS AND CONTRAINDICATIONS
Precautions and contraindications for NPWT are listed in TABLE 15-9. Wounds with low-level vascular compromise may benefit from NPWT and in this situation, research supports the use of intermittent mode therapy at lower pressure levels to reduce the risk of ischemia and ischemic pain.6,7,8,21,30,38 In 2004, Banwell and Musgrave1 reported NPWT showed no benefit for wounds with significant ischemia. This was reported again in 2012 by McCallon45 who stated that NPWT was ineffective on wounds with significant proximal arterial occlusion. NPWT has the potential to increase wound ischemia leading to further tissue necrosis and ischemic pain24 therefore, initiation of treatment is deferred until blood flow is restored by revascularization surgery or endovascular procedures. If blood supply remains marginal, treatment is deferred until signs of granulation appear at the wound edges indicating there is sufficient oxygenation to support angiogenesis. Signs of oxygenation are especially important if there is necrotic tissue in the wound that needs to be debrided before initiation of NPWT.
TABLE 15-9 NPWT Precautions and Contraindications

Precautionary measures are critical when NPWT is placed over exposed named structures (eg, bone, tendon, muscle, organs), grafts, or suture lines.41,42 In most cases, NPWT may be placed over any body tissue46 providing an adequate protective covering is applied prior to application of the wound filler. Several layers of nonadherent Vaseline- or paraffin-impregnated gauze,42 ADAPTIC Non-Adhering Dressing (Systagenix, Quincy, MA), nonadherent silicone or polyester film (Mepitel, Mölnlycke Health Care US, LLC, Norcross, GA),21,47 or white foam are examples of materials to place over fragile and viable structures in order to prevent damage with dressing removal (FIGURE 15-15). Nonadherent contact layers are also used between wound fillers and grafts to protect the fragile new skin from being pulled into the filler via suction forces and also to minimize graft disruption when NPWT dressings are removed.35
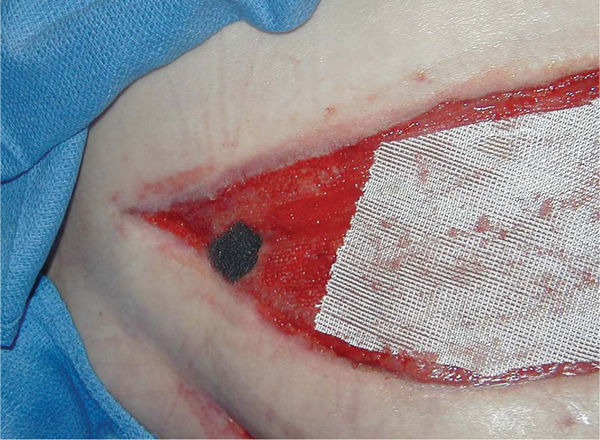
FIGURE 15–15 Protection of granulation tissue Nonadherent mesh is placed over exposed muscle in a post-fasciotomy wound. On the left, a small piece of black foam is placed into a 1.5 cm sinus. Documentation includes all materials placed in the wound (interface materials, types of foam, and number of pieces) to ensure that all materials are removed at the next dressing change.
Pain associated with wound filler removal can be reduced by placing these nonadherent dressings over freshly debrided tissue such as muscle or fascia. Strips of Xeroform (Xeroform Petrolatum Gauze, Covidien/Kendall, Mansfield, MA) or vaseline-impregnated gauze placed over sensitive or inflamed wound edges will decrease pain associated with dressing changes and facilitate epithelial migration once granulation is level with the surrounding skin surface. For all patients, wound tissues are assessed for signs of deterioration at each dressing change (TABLE 15-10) and the lowest effective pressure settings are used that provide the optimal outcome.13,21
TABLE 15-10 Signs of Wound Deterioration13
 Increased local perfusion
Increased local perfusion
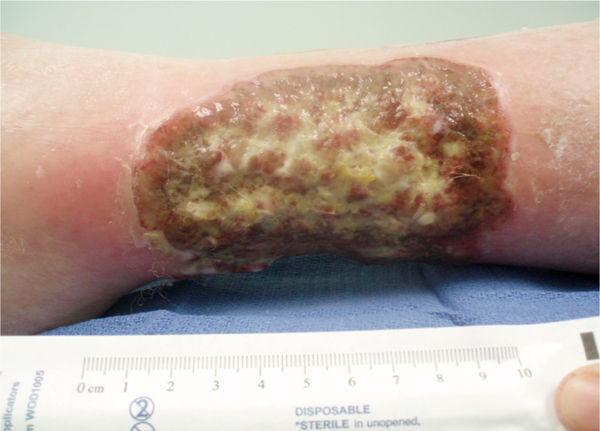
 Increased periwound erythema
Increased periwound erythema






