Introduction582
INTRODUCTION
Fungal identification
SUPERFICIAL FILAMENTOUS INFECTIONS
DERMATOPHYTOSES
Mycology
Specific regional infections
Tinea capitis
Kerion
Favus
Tinea faciei
Tinea barbae, tinea corporis, and tinea cruris
Tinea pedis (athlete’s foot)
Majocchi’s granuloma
Onychomycosis
Treatment of dermatophytoses
Histopathology of dermatophytoses
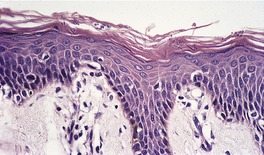
Fig. 25.1
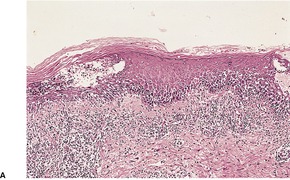
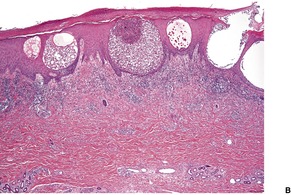
Fig. 25.2
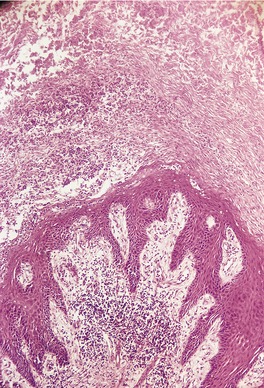
Fig. 25.3
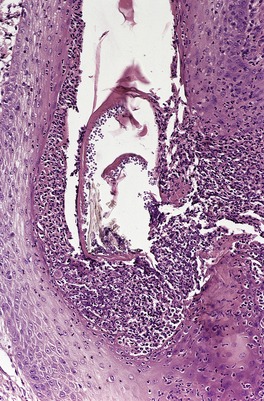
Fig. 25.4
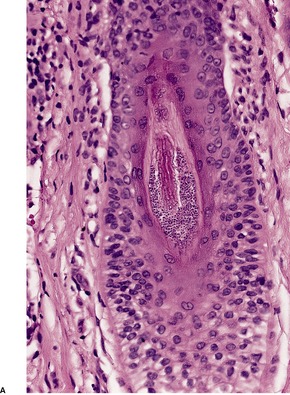
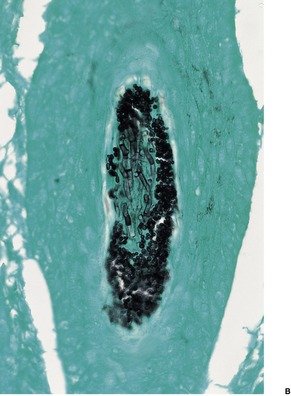
Fig. 25.5
DERMATOMYCOSES
YEAST INFECTIONS
CANDIDOSIS (CANDIDIASIS)
Acute superficial candidosis
Histopathology
Chronic mucocutaneous candidosis
Histopathology
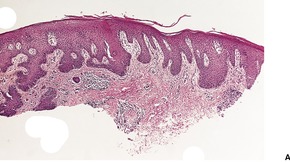
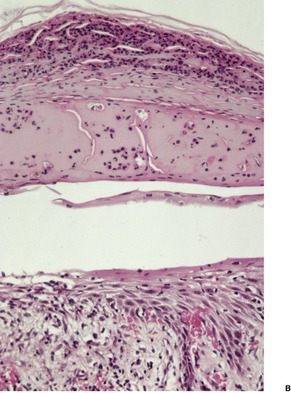
Fig. 25.6
Disseminated candidosis
Histopathology
Candidosis of the newborn
Oral candidosis
Genital candidosis
Periungual candidosis
CRYPTOCOCCOSIS
Histopathology358. and 408.
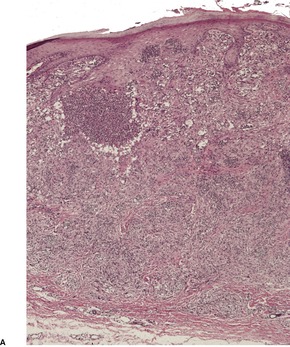
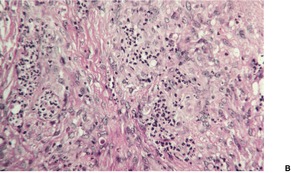
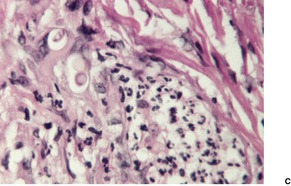
Fig. 25.7
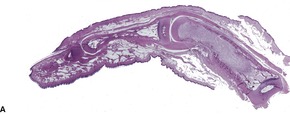
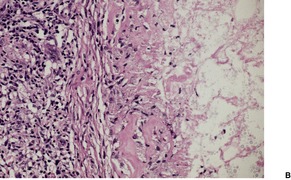
Fig. 25.8
Electron microscopy
PITYRIASIS VERSICOLOR
Histopathology
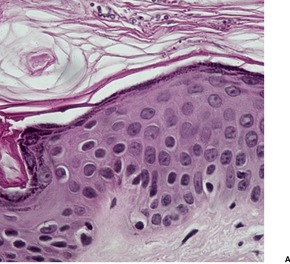
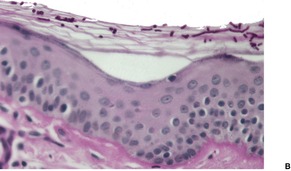
Fig. 25.9
Electron microscopy
PITYROSPORUM FOLLICULITIS
Histopathology461
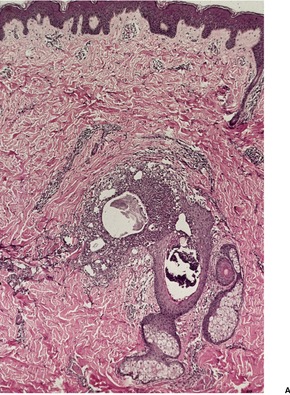
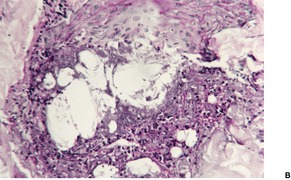
Fig. 25.10
TRICHOSPORONOSIS AND WHITE PIEDRA
Histopathology
SYSTEMIC MYCOSES
(NORTH AMERICAN) BLASTOMYCOSIS
Histopathology484
COCCIDIOIDOMYCOSIS
Histopathology
PARACOCCIDIOIDOMYCOSIS
Histopathology
HISTOPLASMOSIS
Histopathology
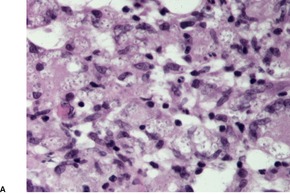
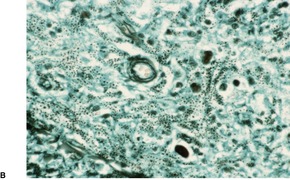
Fig. 25.11
INFECTIONS BY DEMATIACEOUS FUNGI
CHROMOMYCOSIS
Histopathology580. and 583.
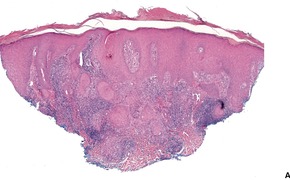
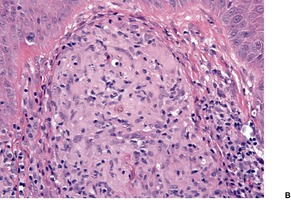
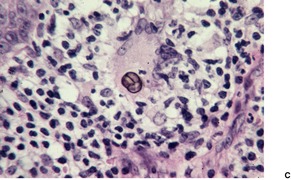
Fig. 25.12
PHAEOHYPHOMYCOSIS
Histopathology660.664.665. and 666.
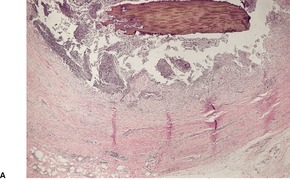
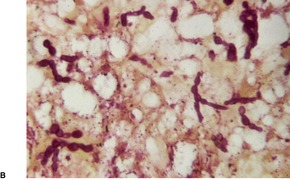
Fig. 25.13
SPOROTRICHOSIS
Histopathology685. and 718.
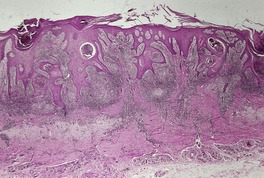
Fig. 25.14
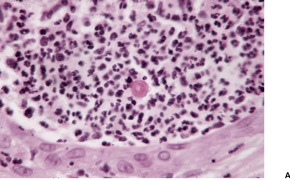
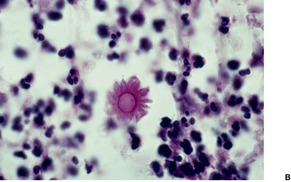
Fig. 25.15
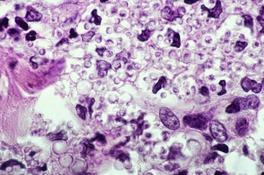
Fig. 25.16
TINEA NIGRA
Histopathology736
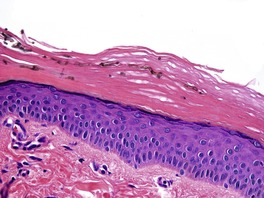
Fig. 25.17
ALTERNARIOSIS
Histopathology746. and 760.
MYCETOMA AND MORPHOLOGICALLY SIMILAR CONDITIONS
MYCETOMA
Macroscopic features of the grains
Eumycetomas
Black grains: Madurella mycetomatis, M. grisea, Leptosphaeria senegalensis, Exophiala jeanselmei, Pyrenochaeta romeroi, Curvularia lunata, Phialophora verrucosa, P. parasitica, Cladophialophora bantiana
Pale grains: Petriellidium boydii, Aspergillus nidulans, A. flavus, Fusarium sp., Acremonium sp., Neotestudina rosatii, dermatophytes
Actinomycetomas
Red grains: Actinomadura pelletieri
Yellow grains: Streptomyces somaliensis
Pale grains: Nocardia brasiliensis, N. cavae, N. asteroides, Actinomadura madurae
Histopathology766. and 771.
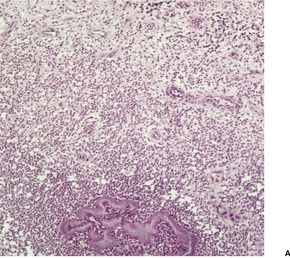
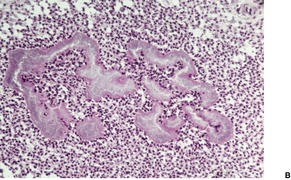
Fig. 25.18
Eumycetomas
Madurella mycetomatis: Large granules (up to 5 mm or more) with interlacing hyphae embedded in interstitial brownish matrix; hyphae at periphery arranged radially with numerous chlamydospores
Petriellidium boydii: Eosinophilic, lighter in the center; numerous vesicles or swollen hyphae; peripheral eosinophilic fringe; other pale eumycetomas have a minimal fringe and contain a dense mass of intermeshing hyphae
Actinomycetomas
Actinomadura madurae: Large (1–5 mm and larger) and multilobulate; peripheral basophilia and central eosinophilia or pale staining; filaments grow from the peripheral zone
Streptomyces somaliensis: Large (0.5–2 mm or more) with dense thin filaments; often stains homogeneously; transverse fracture lines
Nocardia brasiliensis: Small grains (approximately 1 mm); central purple zone; loose clumps of filaments; Gram-positive delicate branching filaments breaking up into bacillary and coccal forms; Gram-negative amorphous matrix (Brown and Benn method)
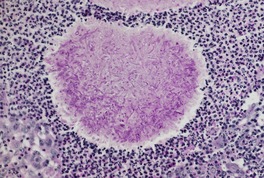
Fig. 25.19
NOCARDIOSIS
Histopathology
ACTINOMYCOSIS
Histopathology853
BOTRYOMYCOSIS
Histopathology866
ZYGOMYCOSES
MUCORMYCOSIS
Histopathology923
SUBCUTANEOUS PHYCOMYCOSIS
Histopathology937.938.939.940.941.942. and 943.
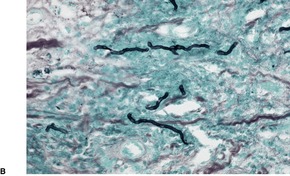
Fig. 25.20
HYALOHYPHOMYCOSES
FUSARIOSIS
Histopathology
PENICILLIOSIS
Histopathology
ASPERGILLOSIS
Histopathology
MISCELLANEOUS MYCOSES
KELOIDAL BLASTOMYCOSIS (LÔBO’S DISEASE)
Histopathology1034. and 1040.
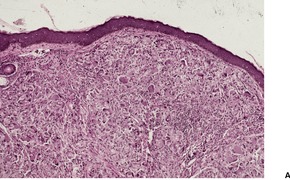
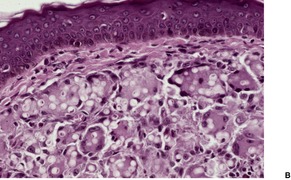
Fig. 25.21
RHINOSPORIDIOSIS
Histopathology1054
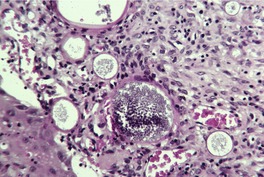
Fig. 25.22
ALGAL INFECTIONS
PROTOTHECOSIS
Histopathology1059
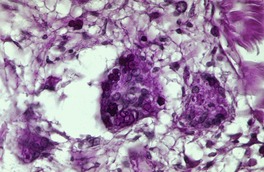
Fig. 25.23
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mycoses and algal infections
The mycoses have long been a confusing area for anyone with only a peripheral interest in mycology. Classifications have been modified repeatedly and some fungi have undergone several changes in nomenclature in the space of a decade. The classification of the various fungal infections of the skin usually takes into account some of the morphological characteristics of the fungus concerned, as well as the distribution and nature of the infection that results. This approach has some shortcomings. For example, tinea nigra could be classified as either a superficial filamentous infection or as a dematiaceous fungal infection. Sporotrichosis is considered in this account with the dematiaceous fungi because of some clinical and histological overlap with chromomycosis. However, the yeast form in tissue sections is not pigmented, although the fungi are dematiaceous in culture. Members of the genus Alternaria are occasionally implicated as agents of phaeohyphomycosis; their colonies are gray to black, although they are not pigmented in tissues. 1 Against this background is the plea for a simplification of the nomenclature and the avoidance of fungal names as part of the clinical nomenclature to avoid confusion when the name of the organism is subject to change as a consequence of taxonomic reclassification. 2 This seems to occur not infrequently. For example, the organism that causes pityrosporum folliculitis is now known as Malassezia.
Fungi grow slowly on laboratory media and their final identification, which is based on the appearance of their colonies and conidia in culture, as well as other characteristics, can take several weeks. 3 Accordingly, direct examination of tissue specimens is often undertaken in conjunction with histopathological examination of biopsy material in order to obtain a more rapid diagnosis.
The most widely used of these techniques, which is of most value in the examination of skin scrapings, is the application of a drop of 10% potassium hydroxide (KOH) to a slide containing the material. This usually clears the tissues in 5 minutes or so, allowing fungi to be more easily visualized. It is our practice to use a solution that combines KOH with glycerol (to prevent drying out) and calcofluor white, an agent that imparts a bright fluorescence to fungi when examined with a fluorescence microscope. If Chicago sky blue 6B is used with KOH as the clearing agent, a standard microscope can be used to examine the scraping. It is particularly useful for the identification of Malassezia furfur, but it can be used for dermatophytoses. 4
There are several methods of identifying fungi in paraffin-embedded material. Many fungi, particularly the dematiaceous and hyaline fungi, are readily visible in sections stained with hematoxylin and eosin. Some difficulty may be experienced with Candida, Cryptococcus, Aspergillus, Blastomyces, Coccidioides, and Mucor. 5 Numerous sections may have to be examined in sporotrichosis to find fungal elements, and in most cases recourse to other stains (see below) is more practical. The dermatophytes are also difficult to see in sections stained with hematoxylin and eosin, but they can sometimes be made visible by racking down the condenser and reducing the light.
Various special stains can be used in an attempt to identify fungi in tissue sections. The PAS stain, sometimes combined with diastase digestion, is most frequently employed. It stains the cell walls of fungi a purple color of varying intensity. The silver methenamine (methenamine silver) stain, usually Grocott’s modification, is a reliable method of detecting fungi: it stains them black against a green background. It is more reliable than the PAS stain for detecting degenerate fungal elements and the rare animal pathogens among the aquatic fungi, although it may be less reliable with zygomycetes. 6 Unfortunately, it stains some structures in inflammatory debris making fungal identification difficult in some circumstances. These two stains have been regarded as broad-spectrum stains because they are positive across a wide range of fungi. 7 Some stains highlight only certain organisms and not others. These so-called narrow-spectrum stains can be used as an adjunct to fungal identification.7. and 8. Cryptococcus neoformans may be stained with the mucicarmine stain or a combined Alcian blue–PAS stain, which shows the cell wall and capsule in contrasting colors. 9 It is usually doubly refractile under polarized light. It also stains with the Masson–Fontana method. These narrow-spectrum stains are discussed further in the relevant fungal disease.
Calcofluor white can be used to stain frozen or paraffin sections as well as tissue smears. 6 The sections must be viewed with a fluorescence microscope. Certain fungi are even autofluorescent when a section stained with hematoxylin and eosin is exposed to ultraviolet light. 5 These include Blastomyces, Cryptococcus, Candida, Aspergillus, Coccidioides, and occasionally Histoplasma.5. and 10.
An antiserum containing a polyclonal antibody to Mycobacterium bovis has been used with immunohistochemical techniques to identify a broad range of bacteria and fungi in paraffin-embedded tissue. This method seems to be particularly useful when organisms are sparse. 11
The use of immunoperoxidase techniques and special fungal antibodies for the detection and diagnosis of fungi in smears and paraffin sections has been described. 12 The fungi stain a golden brown against a pale blue background. The technique is sensitive and specific. A major disadvantage is that most laboratories are unlikely to have available a comprehensive reference collection of fungal antibodies for use in this technique.
Polymerase chain reaction (PCR)-based techniques are now being used to identify specific species of fungi, including dermatophytes.13.14. and 15. They have revealed marked genetic diversity among some fungi, particularly Trichophyton mentagrophytes. 16 Nested PCR is a further accurate method for rapid identification of dermatophytes in clinical specimens. Unfortunately it has a high false negative rate. 17 Real-time PCR assay, using dermatophyte gene-sequence records, is a highly sensitive method for the detection/identification of common dermatophyte infections. 18 PCR amplification combined with restriction enzyme analysis may also be used. 19
The majority of cutaneous mycoses are superficial infections caused by dermatophytes and various yeasts. The dermatophytoses will be considered first.
Two groups of fungal infections are included in this category, the dermatophytoses and the dermatomycoses. They are characterized by the presence of filamentous forms of the organism in tissue sections.
The dermatophytes are a group of related filamentous fungi that have the ability to invade and colonize the keratinized tissues of humans and animals.20.21. and 22. Infections caused by these fungi, which account for 3–4% of dermatological consultations, are known as dermatophytoses (ringworm, tinea).23. and 24.
The clinical appearances are quite variable and depend on a number of factors, including the species of fungus, the site of infection, the immunological status of the patient, and the prior misuse of topical steroids.25. and 26. The usual appearance on glabrous skin is an erythematous (and sometimes vesicular) annular centrifugally growing lesion, with peripheral scale and desquamation and central clearing.23. and 27. Broken hairs and dystrophic nails occur with infections involving these structures. Less common presentations include subcutaneous and deep dermal infections (dermatophytic granuloma, pseudomycetoma)28.29.30.31.32.33.34.35. and 36. and abscesses,37.38.39. and 40. verrucous lesions, 26 granular parakeratosis, 41 blastomycosis-like lesions, 42 and rarely lymphogenous or hematogenous extension. 43 Lymph node involvement may accompany deep dermatophytosis. 44 Immunocompromised individuals are usually involved with these atypical presentations.30.40.45.46.47. and 48. Lesions known as favus, kerion, 49 and Majocchi’s granuloma also occur, and these will be described later. The atypical presentations that may follow the use of topical steroids have been called ‘tinea incognito’.25.50. and 51.
Chronic persisting infections, defined on the basis of duration or treatment failure, also occur. Disturbed cellular immune functions have been found in many of these cases. Diabetes mellitus, palmoplantar keratoderma, 52 ichthyosis, 53 atopic states, collagen diseases, and Cushing’s syndrome may all predispose to chronic and recurrent dermatophyte infections; 54 so too may HIV infection. 55 Despite these findings, a recent study of patients infected with HIV found no increase in the prevalence of uncomplicated dermatophyte infection. 56
A secondary allergic eruption (‘id reaction’) may develop, uncommonly, in patients with dermatophyte infections, particularly tinea pedis. 27 The id reaction (autoeczematization) is usually vesicular and on the palms (see p. 115); it may be more generalized. Erythema nodosum, vasculitis, and erythema multiforme are other rare reactions to dermatophytes. 27
Dermatophytes belong to three genera, Epidermophyton, Microsporum, and Trichophyton. There are three ecological groups of dermatophytes according to their natural habitats: anthropophilic, which preferentially affect humans; zoophilic, in which lower animals are the prime hosts; and geophilic, which live in the soil as saphrophytes.3. and 20. There is geographic variability in the distribution of fungi, although some species are widely distributed throughout the world. 57 The most common isolate is the anthropophilic fungus T. rubrum, which accounts for 40% or more of all dermatophyte infections worldwide.54.58.59. and 60. Other common isolates include T. violaceum61. and 62. (particularly in Africa and Europe, but not America), T. mentagrophytes, T. tonsurans, E. floccosum, M. gypseum,63. and 64. M. canis, and M. audouinii. 65 The last two species are declining in incidence, whereas infections caused by T. rubrum and T. tonsurans are on the increase.57.66. and 67. The zoophilic fungi, such as M. audouinii, M. canis, T. verrucosum, 68 and T. tonsurans, more commonly affect children and tend to evoke a more acute inflammatory response than do the anthropophilic fungi. 27M. canis has been isolated from a neonate in an intensive care unit. 69 In a recent study from Riyadh, Saudi Arabia, T. mentagrophytes and M. canis were the most common dermatophytes responsible for infections. 70
Less common dermatophyte isolates of geographical or occupational interest include T. soudanense71. and 72. (found in Africa and sometimes in travelers), T. concentricum73.74. and 75. (the cause of tinea imbricata in the South Pacific and tropical America), T. erinacei76 (from hedgehogs), M. nanum77 (from swine), T. simii78 (from monkeys), and M. equinum79 and T. equinum80. and 81. (from horses). Mixed isolates, sometimes including a yeast, occur.
The development of infection depends on exposure to an affected source and various factors diminishing host resistance. Associated diseases that predispose to dermatophyte infections have been mentioned above. 54 Local predisposing factors include abrasion, occlusive dressings, sweating, maceration, and poor peripheral circulation. 54 The immunological mechanisms involved in eliminating dermatophytes are poorly understood. Acute infections are associated with good cell-mediated immunity, the short-term development of specific antibodies, and the onset of delayed hypersensitivity. 27 Chronic infections, in which T. rubrum is commonly implicated, 82 are associated with poor in-vitro cell-mediated immunity and sometimes elevated levels of IgE.83.84. and 85. Deep dermal invasion by this organism has also been reported in immunosuppressed patients. 47 The dermatophyte itself may sometimes be the cause of the immunosuppression, which results from a serum factor found in widespread dermatophytosis. 86 Resistance to antifungal therapy does not appear to play a role in these chronic infections. 87 There is no apparent HLA predilection to dermatophyte infections, 88 although it has been suggested that chronic T. rubrum infection (see above) occurs as a specific syndrome involving ‘susceptible’ hosts.53. and 89. Chronic T. rubrum infection has been associated with severe measles in a young female due to suppression of cell-mediated immunity caused by the fungal infection. 90 A study in 2003 concluded that renal transplant recipients were not at increased risk of dermatophytosis, but they were to opportunistic infections with Pityrosporum ovale and Candida albicans. 91
Traditionally, dermatophyte infections of the skin have been considered on the basis of the site of involvement, because there are often some features unique to each. The subtypes considered are tinea capitis (including favus and kerion), tinea faciei, tinea barbae, tinea corporis, tinea cruris, tinea pedis, and onychomycosis. Majocchi’s granuloma is usually considered as a discrete entity. Tinea gladiatorum, found in wrestlers who have close body contact, may result in tinea corporis or tinea capitis; it will not be considered further.92.93.94. and 95. Contact sports, such as judo, are also associated with a higher incidence of dermatophyte infection. 96
Scalp ringworm or tinea capitis has become an increasingly important public health problem in the past decade.97. and 98. It was once almost exclusively an infection of children, and associated with either M. canis or M. audouinii.99.100. and 101. M. canis is the causative organism in only 10% of all tinea capitis infections in the UK. 102 This organism is still common in some countries. 103 Now, T. tonsurans and T. violaceum are the most common isolates in some geographical areas,104.105.106.107.108.109.110.111.112.113.114.115.116.117.118.119.120.121. and 122. causing an endothrix type of hair invasion with the fungus entering the cortex just above the hair bulb and encircling the shaft beneath an intact cuticle. 123 Endothrix infections do not produce fluorescence with Wood’s light, as opposed to ectothrix infections (M. canis and M. audouinii), which give a typical green fluorescence. T. rubrum, the commonest cause of tinea corporis, is not usually regarded as a scalp pathogen, although very occasional cases occur.124.125. and 126. T. soudanese is a common cause of tinea capitis in Africa; it is rare elsewhere except in immigrants from Africa.127.128. and 129. The effects vary from mild erythema with persistent scaliness and minimal hair loss through to inflammatory lesions with pustules and folliculitis and kerion formation.130. and 131. Adults generally present with alopecia and scale, 132 but it may also masquerade as a bacterial pyoderma.133. and 134. Tinea capitis may also mimic dissecting cellulitis. 135 Tinea capitis seems to be surprisingly rare in patients with HIV infection.136. and 137. It is also uncommon in the first year of life.138.139. and 140. Transmission at the hairdresser has been recorded in two elderly women. 141 Household contacts are a potential reservoir of infection. 142 The production of extracellular proteases by the fungi facilitates their dissemination through the stratum corneum of the scalp. 143
A kerion is a boggy violaceous inflammatory area of dermal suppuration and folliculitis.49.144.145.146. and 147. It is most common on the scalp but can be produced in other sites,148. and 149. as an occupational hazard, by zoophilic fungi. T. verrucosum and T. tonsurans, both endothrix fungi, are often implicated in the etiology of a kerion. T. rubrum,150.151. and 152. T. mentagrophytes, 153 and T. erinacei154 are rare isolates. It is the result of a hypersensitivity reaction to the dermatophyte infection. 155 If early treatment is not started, a scarring alopecia may result. 155
Favus is a chronic infection of the scalp, and more rarely of the glabrous skin, which is usually acquired in childhood. 156 It is still found in some developing countries, 112 although its incidence is decreasing in others. 157 The infection can persist for life. It has been associated with disseminated cancer. 158T. schonleini is most commonly involved, and rarely T. violaceum, T. mentagrophytes, or M. gypseum.64.156. and 159. It is characterized by yellow crusts (scutula) overlying an erythematous base. Localized alopecia often results.
Tinea faciei is an uncommon regional variant presenting as a facial erythema with scaling.160. and 161. Diagnosis is often delayed. T. rubrum, 160T. mentagrophytes, 162M. gypseum, 163 and T. tonsurans164 have been implicated. Clinically it may mimic cutaneous lupus erythematosus, rosacea, polymorphic light eruption, granuloma faciale, lupus vulgaris, seborrheic dermatitis, or granuloma annulare.165. and 166. Cases may rarely mimic granuloma faciale167 or cutaneous lupus erythematosus histologically; 165 in another case, abscess formation occurred. 168
Tinea barbae, tinea corporis, and tinea cruris have overlapping clinical features. 169 The usual organisms involved are T. rubrum,170. and 171. T. mentagrophytes, and E. floccosum. 54 Teleomorphs of T. mentagrophytes may also cause tinea corporis. 172 Mixed infections are sometimes recorded. 173E. floccosum particularly involves the groin region and is common in closed communities because it is easily shed. It is unable to invade hair. A kerion-like tinea barbae caused by T. rubrum has been associated with erythema nodosum. 174 A photoexacerbated tinea corporis mimicking subacute lupus erythematosus has also been reported. 175 Tinea cruris occurs almost exclusively in males, and unusual clinical appearances have been noted in patients with AIDS.176. and 177. Penile involvement as an isolated lesion is exceedingly rare. 178 Familial disease has also been reported. 179 Diaper dermatitis is a variant that predominantly affects infants between 7 and 12 months of age. 180Tinea imbricata is a special type of tinea corporis in which there are concentric rings of scale. 181 It is a chronic infection that occurs in the West Pacific and South American regions; it is caused by T. concentricum.73.182. and 183. T. tonsurans and T. mentagrophytes infections may rarely mimic tinea imbricata.184. and 185. The term ‘radiation port dermatophytosis’ is used for cases of tinea corporis localized to irradiated skin. 186
Tinea pedis is the most common regional dermatophytosis in adolescents and adults. T. rubrum and T. mentagrophytes var. interdigitale are the most common isolates.187. and 188. Rare cases of interdigital intertrigo due to species of Fusarium have been reported. 189 The appearances are often modified by maceration and fissuring, but the sharp scaling border is usually preserved. Maceration predisposes to bacterial overgrowth. Once these bacteria propagate, fungi, which initiated the infection, cannot usually be recovered via culture. 190 Tinea pedis is also associated with increased production of the antimicrobial peptide human β-defensin-2, but obviously it does not prevent concurrent bacterial infection in some cases of tinea pedis. 191 Vesicles and pustular lesions are sometimes seen.192. and 193. Tinea pedis is common in swimmers, 194 military personnel, 195 marathon runners, 196 and in some male worshippers who practice communal ablution and subsequent prayer in bare feet. 197 It is increased in hematological malignancies. 198 Unilateral lesions of the sole have been reported in children. 199 Tinea pedis may not be more common in patients with psoriasis, as once thought. 200
Tinea manuum (tinea of the palms) is usually accompanied by tinea pedis and onychomycosis. In the majority of cases, it is caused by T. rubrum, but other organisms have been involved. 201
The term ‘Majocchi’s granuloma’ is given to nodular and plaque-like lesions of the lower leg, most common in females and showing a histological picture of a granulomatous perifolliculitis.202. and 203. Various fungi have been implicated, including T. rubrum, 204M. canis, 202T. violaceum, T. tonsurans,205T. mentagrophytes, and Aspergillus fumigatus. 206 The terms ‘nodular granulomatous perifolliculitis’ and ‘trichophytic granuloma’ have been used for comparable lesions on the calf and scalp, respectively. Trichophytic granulomas, which are often nodular and in the subcutis, may occur in sites other than the scalp. 31 The condition often occurs in immunocompromised individuals.207. and 208.
Onychomycosis is a fungal infection of the nail characterized by thickening, splitting, roughening, and discoloration of the nail. 209 Its incidence is increasing worldwide; its prevalence in Europe may be as high as 26.9%. 210 It accounts for nearly 30% of all superficial fungal infections of the skin and for up to 50% of all onychopathies. 211 Some 50–80% or more cases of onychomycosis are caused by dermatophytes. 212 The remainder result from yeasts, particularly Candida albicans, and various molds, such as Scytalidium dimidiatum, Scopulariopsis brevicaulis, Fusarium sp., Acremonium sp., Alternaria sp., and Aspergillus sp.213.214.215.216.217.218. and 219. Dermatophytes mainly involve the toenails, with T. rubrum and, to a lesser extent, T. mentagrophytes var. interdigitale being the usual agents.209.220.221. and 222. The foot acts as a fungal reservoir; 212 tinea pedis is also present in a third of patients with toenail onychomycosis. 223 Various immunological disturbances and peripheral vascular disease may predispose to infection. Slow nail growth is not a predisposing factor for onychomycosis. 224 Psoriasis appears to constitute a risk factor for dermatophyte infections of the nail but not for all categories of onychomycosis. 225 Onychomycosis is a serious health burden, particularly in older persons and diabetics.226.227.228. and 229. Behavioral factors such as sporting and certain religious practices predispose to onychomycosis. 211 Extensive whitening of the nails due to T. rubrum has been reported in a patient with AIDS. 230T. rubrum infection may also have a genetic basis, with autosomal dominant spread in some families. 231 Children with Down syndrome have a predisposition to this condition. 232
The majority of infections caused by Candida albicans and other species of Candida involve the fingers. The soft tissues around the nail are involved first, producing a paronychia with secondary penetration of the keratin by the fungus.233. and 234. As Candida is not keratolytic it tends to occur in situations of frequent water immersions and immunosuppressed states. 212
Clinically, dermatophyte infections of the nail have traditionally been divided into distal, lateral, proximal, and white superficial variants according to the anatomical localization and appearances. 235 A new classification, expanding on this traditional one, has recently been proposed. 236Its categories are distal and lateral subungual, superficial, proximal subungual, endonyx, and total dystrophic. 236 Fungal infection may also involve the nail isthmus. 237 In superficial white onychomycosis (SWO), invasion of the nail plate is assumed to occur from the dorsal surface. 238 This mode of infection is not universal because SWO can be combined with other categories of onychomycosis. This has led to a new classification system proposing four subtypes of SWO. While the superficial variants are usually due to T. mentagrophytes var. digitale (but not in all countries), 239 the ‘deep’ subtype may be due to molds.209.240. and 241. In the case of proximal subungual onychomycosis, it has been suggested that some cases may be the consequence of lymphatic dissemination of the fungus. 242 A case of this variant caused by M. gypseum has been reported. 243 The occurrence of these different types of SWO appears to reflect differing host–parasite relationships. The variants are not pathogen specific. 209
The fungal elements occur mostly in the deeper portions of the nail plate and in the hyperkeratotic nail bed, rather than on the surface of the nail plate.244. and 245. Sometimes a thick hyperkeratotic nodule forms beneath the nail. This contains numerous clumped hyphae (dermatophytoma).246. and 247. This is an explanation for the negative results obtained from scrapings in some cases of onychomycosis. While mycological culture is the ‘gold standard’ for the diagnosis of onychomycosis, 248 histopathological examination of the nail using the PAS stain is still the most sensitive diagnostic method,249.250. and 251. but the least cost-effective procedure. 252 A potassium hydroxide preparation stained with chlorazol black E is said to be most cost-effective. 252 A recent study from Hideko Kamino’s group in New York has found that the vast majority of cases (97%) of onychomycosis can be diagnosed by histological examination of subungual hyperkeratosis only, avoiding the time-consuming process of softening of the nail plate. 253 As fungi are found by PAS stain in only 60% of morphologically abnormal nails, it is important not to assume that a fungal infection is present in all such nails and commence expensive treatments ‘on spec’.234. and 254. Clinical clues to onychomycosis being the cause of a nail dystrophy include dystrophy confined to the third or fifth toenails on the same foot, unilateral dystrophy, and male gender. 255
The treatment of dermatophyte infections of the skin and nails involves the use of terbinafine, itraconazole, and griseofulvin, with one or other of these therapies proving more effective than others in different regional variants, and with different dermatophytes. Topical therapy can be used for localized cutaneous lesions.
In the case of tinea capitis, griseofulvin was at one time the treatment of choice, but a randomized controlled trial has now indicated that terbinafine, itraconazole, and fluconazole are just as effective. 256 Griseofulvin may be more effective for M. canis infections,47. and 102. although other studies have favored oral terbinafine for this organism. 101 Terbinafine can be used for infections caused by T. tonsurans, and by other species.94.129.257. and 258. Oral itraconazole in three pulses has also been used. 125 Adjunctive antifungal shampoos have been recommended. 256 Despite recommendations that tinea capitis be treated with an oral antifungal agent, only 56% of patients in one study were treated in this way, and only 17% of patients used griseofulvin for the recommended period of at least 6 weeks. 98
In the case of tinea pedis, topical terbinafine was more effective than topical azoles in one study. 256 Oral terbinafine was shown to be effective in another study. Furthermore, this drug seemed to enhance and restore cell-mediated immunity. 259 A recent trial of pramiconazole, a new triazole antifungal agent, found it to be a promising new therapy for tinea pedis and tinea cruris/corporis. 260 In onychomycosis, terbinafine tablets, itraconazole capsules, griseofulvin, and ciclopirox nail lacquer are approved by the FDA in the United States. 256 True superficial white onychomycosis, restricted to the dorsum of the nail, will respond to topical antifungal therapy, but any extension of the infection, and other clinical subtypes will require systemic therapy. 261 One meta-analysis found that systemic terbinafine was better than itraconazole in the treatment of onychomycosis; it was also better than griseofulvin. 262 Another study has found that itraconazole and terbinafine are safe and effective in childhood cases. 263 Other studies have also shown effective treatment with terbinafine. 225 Some of the molds are quite resistant to the usual antifungal drugs. 264 It should be remembered that nails may have a persistently abnormal appearance, even when treatment has been effective. 265 Toenail onychomycosis has been successfully treated with photodynamic therapy. 266
A randomized study of the treatment of tinea imbricata showed that griseofulvin and terbinafine were both effective, while itraconazole, and fluconazole were not. 181
Biopsy material from dermatophyte infections can show a wide range of histological changes. 267 Ackerman has elaborated three different changes in the stratum corneum that can be associated with dermatophyte infections: the presence of neutrophils; 268 compact orthokeratosis (Fig. 25.1); 269 and the presence of the ‘sandwich sign’. 270 The last refers to the presence of hyphae ‘sandwiched in’ between an upper but normal basket-weave stratum corneum, and a lower layer of recently produced stratum corneum which is abnormal in being compact orthokeratotic or parakeratotic in type. 270 The presence of neutrophils in the stratum corneum was not regarded as a reliable sign of dermatophytosis in one study from Vancouver. 271 The presence of neutrophils should always result in the performance of the PAS stain, if it has not already been performed as a routine procedure. Uncommonly, the stratum corneum retains its normal basket-weave pattern.
Dermatophyte infection. Hyphae are present in the compact orthokeratotic layer. (H & E)
The epidermis is often mildly spongiotic; more florid spongiotic vesiculation is usually present when the palms and soles are involved. Subcorneal or intraepidermal pustulation is a less common pattern (Fig. 25.2). 267 Chronic lesions show variable acanthosis (Fig. 25.3). The dermis shows mild superficial edema and a sparse perivascular infiltrate, which includes lymphocytes and occasionally eosinophils or neutrophils.
(A) Dermatophyte infection. (B) Neutrophils are present within the spongiotic vesicle. (H & E)
Tinea imbricata. There are numerous hyphae and some spores in the thickened stratum corneum. The underlying epidermis shows psoriasiform hyperplasia. (H & E)
At times the dermal infiltrate is much heavier, particularly if there is follicular involvement (Fig. 25.4). There may be perifollicular neutrophils or a mixed inflammatory infiltrate (Fig. 25.5). There is a heavy inflammatory infiltrate in a kerion, the proportion of the various cells depending on the duration of the lesion. The changes may vary from a suppurative folliculitis to a granulomatous process. 155 In Majocchi’s granuloma there are perifollicular and dermal granulomas and chronic inflammation; reactive lymphoid follicles may be present. 203 Fungal elements may take several forms: yeasts, bizarre hyphae, and mucinous coatings. 203 Fungal elements are sometimes sparse. 272
Folliculitis with suppuration resulting from a dermatophyte infection. (H & E)
Tinea capitis. (A) There is a sparse perifollicular inflammatory cell infiltrate and numerous fungal elements involving the hair. (H & E) (B) Fungal elements can be seen within the hair shaft (endothrix infection). (Grocott stain)
Rare patterns of inflammation include a resemblance to granuloma faciale, papular urticaria, or eosinophilic pustular folliculitis. 273 In immunocompromised patients, large numbers of hyphae and pseudospores are present in areas of dermal necrosis.26. and 45. The lesions often lack granulomas, a point of distinction from the usual Majocchi’s granuloma (see above).
Dermatophytes exist in tissues in a parasitic form characterized by branched, septate hyphae and small spores. Methods for their identification have been mentioned above. It has been claimed and subsequently refuted that the dendrites of Langerhans cells are PAS positive and can be seen in the stratum corneum of lichenoid dermatitides. 274 The importance of performing a routine PAS stain on all inflammatory skin diseases has been stressed. Hyphal elements were present in one study in only 57% of PAS-positive cases of tinea on H & E sections alone. 275 It is also cost-effective to do routine PAS stains on all inflammatory skin diseases. 276 Automatic staining procedures are easy to establish. A recent study found that the Gomori methenamine silver stain was superior to the PAS stain for the routine diagnosis of onychomycosis. 277
Dermatophytes may invade the hair shaft (endothrix infection) or remain confined to its surface (ectothrix infection). T. tonsurans, T. violaceum, and T. soudanense are true endothrix parasites. 278
The term ‘dermatomycoses’ encompasses infections of the hair, nails, or skin caused by non-dermatophytes which have filamentous forms in tissues. It covers such infections as tinea nigra, piedra, pityriasis versicolor, and candidosis.
Infections caused by the molds Scytalidium dimidiatum (the anamorphic form of Nattrassia mangiferae, formerly known as Hendersonula toruloidea)279. and 280. and Scytalidium hyalinum281 are included as dermatomycoses. They are increasingly important as a cause of onychomycosis and tinea pedis.264.282. and 283. They have been reported in patients with coexistent systemic disease such as lupus erythematosus, diabetes mellitus, cancer, 284 and dyskeratosis congenita. 285 They are being isolated with increasing frequency in the United Kingdom and Europe from individuals who have emigrated from tropical regions.264. and 286. There is no orally effective treatment. 283
Scopulariopsis brevicaulis, a widespread saprophytic fungus, is included here for completeness. It has been associated with onychomycosis and rarely chronic granulomatous skin infections.287. and 288. Saccharomyces cerevisiae (baker’s yeast) is an exceedingly rare cause of systemic and/or cutaneous infection in immunocompromised patients. 289 It responded in one case to voriconazole. 289 Systemic infections with these various organisms have been treated in the past with amphotericin B and fluconazole.
Yeasts are fungi of primarily unicellular growth habit. 290 The normal vegetative cells of yeasts are round or oval and measure 2.5–6 µm in diameter. Many yeasts can form hyphae or pseudohyphae in cutaneous infections. They are a regular constituent of the normal human flora, but most are potential pathogens. Opportunistic yeast infections increased with the advent of broad-spectrum antibiotics and immunosuppressive therapy. 291 They are now an important complication in patients with AIDS.
Candida albicans and Cryptococcus neoformans are the most important yeasts, producing, respectively, candidosis and cryptococcosis.
Malassezia globosa, previously known as M. furfur and Pityrosporum orbiculare, produces the cosmetically disfiguring condition pityriasis versicolor (tinea versicolor). Malassezia can also produce folliculitis. It has been incriminated in the etiology of confluent and reticulated papillomatosis, and of some cases of seborrheic dermatitis (including its occurrence in patients with AIDS), 292 dandruff, psoriasis, and atopic dermatitis.293.294. and 295. It is likely that the organisms are present because of the favorable ‘soil’ in these conditions.
Trichosporon sp. can produce both white piedra, a superficial infection of the hair, and a disseminated infection in immunosuppressed patients. It is discussed later in this section.
The genera Rhodotorula, 291Torulopsis, 291 and Sporobolomyces296 are of little importance in relation to the skin and will not be considered further.
Candida albicans is the most frequent species of Candida implicated in human infections. These range from relatively trivial superficial infections to fatal disseminated disease.297.298. and 299. Candida albicans is a normal inhabitant of the gastrointestinal tract and is found in the mouths of 40% of normal individuals. It is sometimes isolated from the skin surface, but it is not a usual constituent of the skin flora. There are many factors that predispose to clinical infection. These include pregnancy, 300 the neonatal period, immunological and endocrine dysfunction, antibiotic therapy, and immunocompromised and debilitating states. 297 Local factors such as increased skin moisture and heat also play a role. Recent studies have provided a better understanding of the various factors that contribute to the invasive properties of Candida albicans. 301 For example, the yeast can express at least three types of surface adhesion molecules to colonize epithelial surfaces, plus an aspartyl proteinase enzyme which facilitates penetration of keratinized cells. 301
A number of clinical variants of candidosis occur: acute superficial candidosis, chronic mucocutaneous candidosis, systemic (disseminated) candidosis, and candidosis in the infant. 297 Oral, periungual, and genital candidosis are best regarded as distinct entities that may occur alone or in association with other clinical forms of candidosis. Folliculitis and delayed surgical wound healing are rare manifestations of Candida infection.302. and 303. Candida folliculitis may mimic tinea barbae.304. and 305. Another species of Candida, C. parapsilosis, can sometimes cause localized and systemic infections in immunocompromised patients, and after extensive burns. It has also been responsible for a chondritis after surgery to the ear. 306
Acute superficial candidosis is the usual form of cutaneous infection with Candida species. There are vesicles, pustules, and crusted erosions with a beefy-red appearance. These develop on skin folds and other areas, particularly in individuals living in a humid environment. 297 The condition may be self-limited; it responds well to treatment. Decubital candidosis is a variant of cutaneous candidosis that occurs on the dorsal skin of chronically bedridden patients; often there has been long-term use of antibiotics. It does not seem to predispose to disseminated (systemic) candidosis. 307
The characteristic histological feature is the presence of neutrophils in the stratum corneum. The infiltration may take the form of small collections of cells, spongiform pustulation, or subcorneal pustulation resembling impetigo. The underlying epidermis may show focal spongiosis and mild acanthosis. Fungal elements may be sparse. They are best visualized with the PAS stain. They can also be demonstrated with the Gomori methenamine silver stain, but not the Congo red stain. 8 Mycelia predominate over spores. Electron microscopy has shown that the majority of the fungal elements are inside the epithelial cells.308. and 309.
The term ‘chronic mucocutaneous candidosis’ covers a heterogeneous group of disorders characterized by chronic and persistent infections of the mucous membranes, and infections of the skin and nails by various species of Candida, usually C. albicans.310.311.312. and 313. The condition ranges in severity from a mild localized and persistent infection of the mouth, nails, or vulva to a severe generalized condition. 297 It may be associated with a spectrum of cellular immunodeficiency states, including several defined syndromes that range from life-threatening to subtle. 311 A deficiency of the cytokine interleukin-2 was present in one case. 314 Other cytokines are also involved, and it now appears that the basic defect is altered cytokine production in response to candida antigens. 315 Other associations include endocrinopathies and nutritional deficiencies, the latter including disorders of iron metabolism.297.311. and 316. On the basis of recent cases, it appears that there are two Candida endocrinopathy syndromes, one associated with hypoparathyroidism and/or hypoadrenalism,317. and 318. and the other associated with hypothyroidism. The former is inherited as an autosomal dominant trait and the latter syndrome as an autosomal recessive. 319 This inheritance is at odds with the OMIM gene map that lists chronic mucocutaneous candidosis with thyroid disease (OMIM 606415) as an autosomal dominant condition linked to chromosome 2p. Candidosis with hypoparathyroidism and/or Addison’s disease (OMIM 240300) is now called the autoimmune polyendocrine syndrome, type 1, and is caused by a mutation in the autoimmune regulator gene (AIRE) linked to chromosome 21q22.3.320. and 321. Two further familial chronic mucocutaneous candidosis syndromes include an autosomal dominant form without endocrine disease (OMIM 114580) and a very rare form with only nail candidosis, and intercellular adhesion molecule-1 (ICAM-1) deficiency (OMIM 607644). 322 Late onset of chronic mucocutaneous candidosis in adults is rare and usually associated with cancer, particularly a thymoma.311. and 323. It has also been associated with hyperimmunoglobulin E syndrome. 324 Agammaglobulinemia is a further recently described association. 325
In all clinical groups, vaginitis, paronychia, and oral thrush may also be present. The cutaneous lesions are asymptomatic plaques on the dorsum of the hands and feet and periorificial skin. 323 They are brown-red with sharp margins and a soft scale. 323 Sometimes a more extensive scaling eruption is present. Nail dystrophy may occur.326. and 327. Granulomatous lesions have been recorded. 328 In 20% of all cases there is a concurrent dermatophyte infection.316. and 323. Antigliadin antibodies were present in one case. 329
Because of the chronicity of the condition, a diverse range of topical and oral antifungal treatments has been used. Azole antifungal agents (ketoconazole, itraconazole, and fluconazole) are often used, but recurrence of the disease occurs after cessation of the drug.327. and 329. Amphotericin B was used prior to the introduction of azole drugs.
There is some histological resemblance to the acute form, although the lesions tend to have more epidermal acanthosis, sometimes being vaguely psoriasiform in type (Fig. 25.6). There may be areas of compact orthokeratosis269 and others of scale crust formation with degenerating neutrophils. This reflects the chronicity of the lesions. Spores and hyphae are usually found without difficulty in PAS preparations.
Chronic candidosis. (A) There is mild psoriasiform hyperplasia of the epidermis. (B) There is overlying scale crust containing degenerate neutrophils. (H & E)
In granulomatous lesions there are vaguely formed granulomas in the dermis composed of lymphocytes, plasma cells, epithelioid cells, and occasional Langhans giant cells. Occasional yeast forms and pseudohyphae may be found in the granulomas.
Disseminated (systemic) candidosis is increasingly being recognized in immunosuppressed and debilitated patients, and in patients with hematological disorders and neutropenia, particularly those with central venous catheters and those receiving broad-spectrum antibiotics.297.330.331.332. and 333. Multisystem involvement occurs, although cutaneous lesions are present in only 15% of cases. 331C. tropicalis is a frequent isolate from the cutaneous lesions in this type of candidosis.333. and 334.
There is an erythematous papulonodular rash, with multiple lesions on the trunk and proximal parts of the extremities. The face and distal extremities may also be involved. 333 Sometimes only isolated lesions are present. Other rare clinical presentations mimic ecthyma gangrenosum,335. and 336. and leukocytoclastic vasculitis. 337 The mortality rate in one series was 84.2%. 333
Systemic candidosis is well recognized in heroin addicts, but only comparatively recently have cutaneous lesions, in the form of folliculitis, been reported in some addicts.338. and 339.
Treatment with amphotericin B and/or fluconazole has been used.
There are small microabscesses in the upper dermis, sometimes centered on blood vessels. 331 A few budding yeasts may be found in these areas on a PAS stain. 331 At other times the reaction is much milder, with only a perivascular mixed inflammatory cell infiltrate. A leukocytoclastic angiitis has been reported. 334 In lesions resembling ecthyma gangrenosum, the papillae are edematous and distended by numerous pseudohyphae, which may extend into vessel walls. 336 Ulceration is also present. In heroin addicts there is a suppurative folliculitis and perifolliculitis. Pseudohyphae are sometimes found within the hair.
There are several distinct clinicopathological entities within this group: congenital cutaneous candidosis, neonatal candidosis, and infantile gluteal granuloma. 297 Immunity is not impaired.
Congenital cutaneous candidosis presents at birth or in the first days of life with generalized erythematous macules and papulopustules.340. and 341. It results from intrauterine infection.342. and 343. Organisms may be demonstrated in the placenta and in the stratum corneum of the neonatal lesions. 340
Neonatal candidosis presents with oral and perioral lesions in the first 2 weeks of life. 342 Infection is probably acquired during intravaginal passage at the time of delivery. 342 Sometimes there is involvement of the diaper area. 342 Severe, so-called ‘invasive fungal dermatitis’ is a rare form of cutaneous fungal infection occurring in neonates weighing less than 1000 grams. 344
Infantile gluteal granuloma is an etiologically controversial entity characterized by discrete granulomatous lesions in the diaper (napkin) area. 345 Diaper dermatitis is part of the spectrum.346. and 347. The role of Candida is uncertain.297.345. and 348. The use of topical fluorinated steroids and plastic pants in infants with diaper dermatitis has been incriminated.345. and 349. Rarely, a similar entity has been reported in this region in adults, possibly as a consequence of Candida infection. 350
Oral candidosis (thrush) is found mostly in infants as irregular white patches and plaques.297. and 351. It can also be found as part of chronic mucocutaneous candidosis and in debilitated adults on long-term antibiotics or with a hematological malignancy. Rarely, thrush is related to poor oral hygiene and dentures. 352 Oral candidosis has been reported as an initial manifestation of the acquired immunodeficiency syndrome (AIDS). 353
Other patterns of mucosal involvement occur on the tongue. These include median rhomboid glossitis, 354 and black hairy tongue, although the latter has been attributed to species of Candida other than C. albicans. 297 A perioral pustular eruption has been ascribed to Candida. 355
Epithelial hyperplasia is a characteristic feature of mucosal infection.
Paronychia may occur as an isolated infection, particularly in women who frequently immerse their hands in water. 357 Minor mechanical trauma, diabetes, and circulatory disturbances may also be incriminated. 297 The nail of the middle finger of the dominant hand is most frequently involved. 357 In chronic mucocutaneous candidosis there is usually onychodystrophy with nail bed deformity rather than onycholysis, which is more often a manifestation of acute infection.
Cryptococcus neoformans (formerly known as Torula histolytica) is an encapsulated yeast-like fungus found in dried avian (particularly pigeon) and bat excreta, and in dust contaminated with such droppings.358.359.360.361. and 362. Its usual portal of entry is the respiratory tract, leading to the formation of pulmonary granulomas. Meningoencephalitis is another clinical presentation of cryptococcosis. Hematogenous dissemination leading to cutaneous involvement occurs in about 10–15% of cases of cryptococcosis. Cryptococcus laurentii is a rare human pathogen. 363
Skin lesions may be the first evidence of an occult systemic infection.364. and 365. Although many cases have been reported as instances of primary cutaneous cryptococcosis, 366 probably only a few of these have resulted from primary inoculation of organisms into the skin, thereby fulfilling the criteria of a true primary cutaneous infection.367.368.369.370. and 371. Preceding trauma has sometimes been implicated in primary cutaneous disease.372. and 373. Most patients are immunocompromised,374.375.376.377.378.379.380.381. and 382. and the infection is now a well-recognized occurrence in patients with AIDS.383.384.385.386.387.388.389.390. and 391. With improving antiretroviral therapy for patients with AIDS, organ transplant recipients are now at the highest risk of acquiring cryptococcosis. The risk in transplant recipients is 2.6–2.8%.392. and 393. The new immune modulator drugs have recently been associated with localized and disseminated disease.392.394. and 395. The cutaneous presentations of cryptococcosis are protean and include papulonodules, ulcers, pustules, plaques, ecchymoses, and cellulitis.396.397.398. and 399. Lesions may rarely simulate pyoderma gangrenosum, 400 herpes,383. and 401. keloids, 402 or molluscum contagiosum.385.386.401. and 403. Any site may be involved, but there is a predilection for the face, neck, and forearms. 370
Concurrent infection with alternariosis has been reported. 404 In another case, leprosy was the associated infection. 405
A rapid diagnosis may be made by examination of India-ink preparations of aspirates or Tzanck smears. 392 The organisms are readily isolated on Sabouraud’s agar. Rarely, other species of Cryptococcus have been isolated from infected tissues.406. and 407.
The histology is variable, ranging from tuberculoid granulomas in the dermis and upper subcutis, with few organisms, to lesions in which large numbers of the yeast-like organisms, surrounded by their mucinous capsular material, form extensive mucoid masses (Fig. 25.7 and Fig. 25.8). 374 The organisms mostly range from 5 to 15 µm in diameter. A common pattern is a dense infiltrate of chronic inflammatory cells with multinucleate giant cells containing several organisms with refractile walls. Focal granulomas may be present, as may some small spaces containing numerous organisms, both free and in macrophages. Palisaded granulomas are rare. 409 A few neutrophils are often present; small microabscesses are less common. The overlying epidermis may show acanthosis, mild pseudoepitheliomatous hyperplasia, or ulceration. Transepidermal elimination of organisms may be seen. 358
Cryptococcosis. (A) There are granulomas and sheets of inflammatory cells in the dermis. (B), (C) There are numerous yeasts in macrophages and giant cells. (H & E)
Cryptococcosis. (A) Extension into bone and joint is occurring in this amputated finger. (B) There is a mucinous area with numerous organisms and a surrounding inflammatory palisade. (H & E)
Cryptococcal inflammatory pseudotumors have been described in HIV-positive patients. 410 There was a storiform arrangement of spindle cells, in addition to spindle and polygonal cells that were arranged in a haphazard manner. 410 There were scattered lymphocytes, plasma cells, and giant cells. Focal necrosis and some fibrosis were also present. Immunophenotyping of the spindle cells showed a mixed histiocytic and myofibroblastic lineage, with a predominance of histiocytes. 410
The cell wall of C. neoformans will stain with the PAS or silver methenamine methods, and the capsule with mucicarmine or Alcian blue. 358 A combined PAS–Alcian blue stain which contrasts the cell wall and capsule is a useful method. The Alcian blue staining of Cryptococcus is shared only with Pityrosporum, and to a lesser degree with Blastomyces. 7Cryptococcus does not stain with Congo red, as occurs with Pityrosporum. 7 Phagocytosed organisms often have an attenuated capsule that does not stain. Non-encapsulated tissue variants411 and hyphal forms have been described in other sites. The latter are found only very rarely, and usually only in superficial ulcerated lesions at the body orifices. The organism is usually doubly refractile under polarized light. It may be confirmed by the use of indirect immunoperoxidase methods on routine paraffin sections. 412 Typing of the isolate by PCR fingerprinting using available primers can also be performed. 413
The organisms have an electron-dense wall and a surrounding clear space, beyond which is the capsule. 374 In some phagocytosed organisms the capsule has been destroyed and there is only a small amount of fibrillary material remaining. 408 Projecting buds may be seen, even on phagocytosed organisms.408. and 414.
Pityriasis versicolor (tinea versicolor) is a relatively common non-contagious superficial fungal infection, usually located on the upper trunk or upper arms.415.416. and 417. It is both chronic and recurrent. Its prevalence in a group of young Italian sailors was 2.1%, 418 while in a group of Italian pregnant women it was 5.7%, which was said to compare with a 2–5% prevalence in temperate climates. 419 Lesions are slightly scaly and may be macular, nummular, or confluent. They vary in color from red-brown to white, and the scales show yellow fluorescence with Wood’s light. 415 Infections are more common in patients with seborrheic dermatitis, dandruff, 420 or hyperhidrosis, and in residents in the tropics. 414 Involvement of the nipple has been attributed to the increased concentration of sebaceous glands in that region. 421 Increased sweating may account for the occurrence of lesions in the flexures. 422 In infants, a papulopustular eruption of the cephalic area (neonatal acne) may result from infection with the same fungus. 423 Colonization of neonate skin by species of Malassezia is common (30% at 2–4 weeks of age) so that care must be taken in attributing any neonatal disease to its presence. 424 It is now thought that it does not cause neonatal cephalic pustulosis (neonatal acne), 424 despite earlier reports suggesting an association. 425 A rare atrophic variant of pityriasis versicolor, at times mimicking mycosis fungoides or anetoderma clinically, has been reported by Crowson and Magro. 426 Prior corticosteroids had been used in only one of the 12 cases they reported. The pathogenesis of these lesions is unclear. Lesions resembling pityriasis rotunda (see p. 255) have also been reported. 427 In another case, overlapping parallel scales resembling tinea imbricata were present. 428 One subgroup of atopic dermatitis (head and neck dermatitis) can be aggravated by Malassezia sp.429. and 430. Although affected patients have a normal cell-mediated immune response to the organism, they do not generate a protective response to mycelial antigens. 431 Finally, pityriasis versicolor has been associated with the use of etanercept therapy. 432
The causative organism, previously called Malassezia furfur, is a dimorphic lipophilic fungus that is a normal inhabitant of the stratum corneum and infundibulum of the hair follicle.433.434. and 435. The yeast phase of this organism has two morphologically discrete forms: an ovoid form, known for decades as Pityrosporum ovale, and a spherical form, Pityrosporum orbiculare. 436 Each form can transform into the other. A taxonomic revision of the genus Malassezia was carried out in 1996 with the description of four new species: M. globosa, M. restricta, M. obtusa, and M. slooffiae. M. sympodialis and M. pachydermatis had been described earlier, the latter being regarded as a resident of animal skin. 437 Since that time, three more species have been appended to the genus: M. dermatis, M. japonica, and M. nana. 294 The following summary details our current knowledge. It is likely to change as more studies accumulate from both tropical and temperate climates.
• M. globosa – pityriasis versicolor and healthy skin
• M. sympodialis – normal skin, particularly trunk
• M. restricta – seborrheic dermatitis and dandruff295
• M. pachydermatis – cats and dogs, systemic infection in premature infants
• M. slooffiae – normal skin, low number of isolates
• M. obtusa – rare isolate, little known about it
• M. dermatis – isolated from patients with atopic dermatitis in Japan
• M. japonica – as for M. dermatis
• M. nana – an animal isolate.
As a consequence of recent studies it now appears that pityriasis versicolor is caused by M. globosa in its mycelial phase. 437
Hypopigmentation in pityriasis versicolor (pityriasis versicolor alba) results from the production of dicarboxylic acids by the organisms. 438 These have a tyrosinase inhibitor effect, thus interfering with the synthesis of melanin.439. and 440. Confetti-like hypopigmentation has been reported. 441Hyperpigmentation may result in part from the production of large melanosomes, singly distributed, 442 but also from vascular hyperemia, orthokeratosis, and the presence of organisms.443. and 444.
Pityriasis versicolor may be treated with topical or oral antifungal agents, the latter being used for widespread disease or failed topical therapy. 445 The triazoles (itraconazole, ketoconazole, and fluconazole) are often used in therapy. 445 Successful treatment of the disease using photodynamic therapy with 5-aminolevulinic acid has been reported. 446
There is slight to moderate hyperkeratosis and acanthosis. The dermis contains a mild, superficial perivascular inflammatory infiltrate which includes lymphocytes, histiocytes, and occasional plasma cells. There may be mild melanin incontinence in some cases. In the stratum corneum there are numerous round budding yeasts (blastoconidia) and short septate hyphae (pseudomycelium), giving a so-called ‘spaghetti and meat balls’ appearance (Fig. 25.9). Pityrosporum are clearly seen in H & E, and PAS preparations. They also stain with Alcian blue (as does Cryptococcus), Congo red (as does Blastomyces), and the Masson–Fontana stain (as does Cryptococcus). 7 They are not acid fast.
Pityriasis versicolor. (A) Numerous budding yeasts and short hyphae are present in the stratum corneum. (H & E) (B) They can also be seen with special stain. (PAS stain)
One study has shown that helper-inducer T cells dominate among the sparse dermal infiltrate. 447 These cells may be responsible for the atrophic lesions described by Crowson and Magro (see above) by producing cytokines that interfere with collagen metabolism and/or keratinocyte growth. The lesions described in this report showed variable epidermal and dermal atrophy with effacement of the rete ridges, subepidermal fibroplasia, pigment incontinence, and elastolysis. 426
Fungi can be demonstrated at all levels of the stratum corneum, in follicles, and even intracellularly. 448
Pityrosporum folliculitis presents as erythematous follicular papules and pustules, 2–4 mm in diameter, with a predilection for the upper back, shoulders, chest, and upper arms.415.449. and 450. The lesions can be quite pruritic. 451 It is more common in females, and in those over the age of 30. 452 Sometimes there is associated seborrheic dermatitis or pityriasis versicolor.449. and 453. It has been reported in Down syndrome, 454 pregnancy, 455 and in immunocompromised patients,456. and 457. in whom it may be confused clinically with more serious infections. 458 It has been reported as a nosocomial infection in three patients in the same intensive care unit. 459Malassezia furfur can be cultured from lesions in about 75% of cases, 449 and affected individuals have serum antibody titers against this organism. 453 Using the most recent taxonomic classification, it is likely that M. globosa is the organism responsible. There is some evidence to suggest that follicular occlusion is the primary event in the pathogenesis of this condition, with yeast overgrowth being a secondary occurrence. 460
Involved follicles are dilated and often plugged with keratinous material and debris. There is a mild chronic inflammatory cell infiltrate around the infundibular portion of the follicle. Intrafollicular deposits of mucin are sometimes present. 462 If serial sections are examined, disruption of the follicular epithelium is sometimes found, with basophilic granular debris, keratinous material, neutrophils, and other inflammatory cells in the perifollicular dermis (Fig. 25.10). 461 A few foreign-body giant cells may also be present when rupture of the follicle has occurred. A PAS or silver methenamine stain will reveal spherical to oval yeast-like organisms, 2–4 µm in diameter. These organisms are sometimes budding. They are found most often in the follicle, but following rupture they can also be found in the perifollicular inflammatory exudate. 463 Sometimes a few hyphae can also be seen. 449 Pseudoactinomycotic granules have been reported in two cases. 464
Pityrosporum folliculitis. (A) Inflammatory cells and basophilic granular debris are present in the dermis adjacent to the point of rupture of the hair follicle. (H & E) (B) The tiny yeasts can just be seen at this magnification. (PAS stain)
The yeast Trichosporon asahii (formerly called T. beigelii and T. cutaneum) is a rare cause of a generalized blood-borne infection in immunosuppressed patients, 465 particularly those with leukemia or a lymphoma.466. and 467. Trichosporonosis is frequently fatal in this clinical setting. 466 Disseminated infection with Trichosporon inkin and Histoplasma capsulatum has been reported in a patient with newly diagnosed AIDS. 468 Cutaneous lesions occur in approximately 30% of patients with this infection; lesions take the form of purpuric papules and nodules with central necrosis or ulceration. 465 Isolated skin lesions and hand eczema are exceedingly rare.469.470.471. and 472. Chronic cutaneous infection with T. asahii has been reported in a non-immunocompromised person. 473 In one instance, a cutaneous abscess due to this organism occurred at the site of corticosteroid injection into a hypertrophic scar. 474T. asahii is a common cause of onychomycosis and tinea pedis in some countries.475. and 476.
A reclassification of the genus Trichosporon has taken place; the ‘old’ T. beigelii has been replaced by at least six species, one of which is T. asahii. 470T. ovoides and T. inkin are the species now considered responsible for white piedra, a rare superficial infection of the hair resulting in white to tan-colored gritty nodules, just visible to the naked eye, along the hair shaft.477. and 478. The scalp, face, or pubic area may be involved. White piedra must be distinguished from black piedra, in which tightly adherent black nodules form on the hair, particularly on the scalp. 479 Black piedra is caused by infection with Piedraia hortae, which is not a yeast but an ascomycete. Piedra is discussed further in Chapter 15 (p. 416).
Isolated cutaneous lesions due to T. asahii have been treated successfully with oral itraconazole and topical tioconazole cream. 472
In fatal systemic infections numerous slender hyphae and budding yeasts can be seen in the deep dermis and in the walls of blood vessels.466. and 480. The inflammatory response is usually poor because of the underlying neutropenia. 466
In the localized cutaneous form, chronic inflammation with granuloma formation occurs in the mid and deep dermis, often with extension into the subcutis.465. and 469. Numerous fungal elements can usually be seen on the PAS stain.
In white piedra, discrete nodules are found at intervals along the hair shaft. High-power light microscopy shows that the nodules consist of numerous spores. Scanning electron microscopy has shown hyphae perpendicular to the surface which are overlaid by budding arthrospores. 481 In black piedra, masses of brown hyphae with ovoid asci containing 2–8 single-celled ascospores are present along the hair shaft (see p. 416). 479
The term ‘systemic mycoses’ is used here to refer to infections caused by organisms in the following genera: Blastomyces, Coccidioides, Paracoccidioides, Histoplasma, and Cryptococcus. In most cases the infection develops initially in the lungs; later, the skin and other organs may be involved. All these organisms except Cryptococcus neoformans are dimorphic, growing as mycelia in their natural state and assuming a yeast form in tissues. Cryptococcosis has already been considered with the infections caused by yeasts, and will not be considered further in this section. There are several reports of Chrysosporium parvum, a filamentous soil saprophyte, producing pulmonary disease and a localized cutaneous disease. 482 It may mimic, histologically, the other diseases included in this section. The dematiaceous fungi have also been excluded from this group.
Patient prognosis in these conditions is influenced by a timely diagnosis and commencement of treatment. 483 The diagnostic gold standard, tissue culture, may take days or weeks to complete. Often, tissue is not even submitted for culture as an infective etiology is not considered likely on clinical grounds. In-situ hybridization using oligonucleotide probes directed against fungal ribosomal RNA is a rapid method of making a specific diagnosis using paraffin-embedded tissue. 483
(North American) blastomycosis, caused by Blastomyces dermatitidis, occurs on the North American continent and in parts of Africa.484. and 485. Within the United States, most cases are concentrated along the Mississippi, Missouri, and Ohio River basins and the Great Lakes. 486 It has also been reported in India. 487 There are three clinical forms: pulmonary blastomycosis, disseminated blastomycosis, and a primary cutaneous form that results from direct inoculation of organisms into the skin.484.488. and 489. Most cutaneous lesions occur in the course of disseminated disease (secondary cutaneous blastomycosis); in this form the lesions may be restricted to the lungs, skin, and subcutaneous tissue.486.490. and 491. The rare primary inoculation form may be followed by lymphangitic lesions comparable to those of sporotrichosis. 484 Distinguishing between primary and secondary cutaneous involvement is sometimes difficult. 492 The more usual primary lesion is a crusted verrucous nodule, sometimes with central healing and scarring, or an ulcerated plaque.490. and 493. Multiple lesions are sometimes present. 494 A widespread pustular eruption has been reported; 495 it resembled Sweet’s syndrome in one patient. 496 The disease is more frequent in adult males. Cases have been reported in children, both immunosuppressed and immunocompetent.494. and 497. It shows a predilection for exposed skin, particularly the face.
Treatment of blastomycosis is usually with amphotericin B, fluconazole, or itraconazole.486.493. and 494. Oral itraconazole has been used to treat most recently reported cases of cutaneous disease.
An established verrucous lesion has many histological features in common with chromomycosis and sporotrichosis. There is pseudoepitheliomatous hyperplasia and a polymorphous dermal inflammatory cell infiltrate with scattered giant cells. Microabscesses are characteristic and occur in the dermis and in acanthotic downgrowths of the epidermis. Poorly formed granulomas and suppurative granulomas may be present. In the case resembling Sweet’s syndrome (see above), there was a diffuse dermal infiltrate of neutrophils, without epidermal hyperplasia. 496 There were scattered histiocytes and giant cells containing budding organisms. 496
The thick-walled yeasts measure 7–15 µm in diameter; they are found in the center of the abscesses and in some of the giant cells. A single bud is sometimes present on the surface of the organism. Giant yeast forms, some greater than 40 µm in diameter, within and surrounding vessels, have been reported in immunosuppressed patients. 498 If organisms are difficult to find in hematoxylin and eosin-stained sections, a PAS or silver methenamine stain will usually demonstrate them. Cases have been misdiagnosed initially as cryptococcosis, but C. neoformans is usually slightly smaller, and more numerous in the tissue, than Blastomyces. Furthermore, it has a mucinous capsule, not seen in Blastomyces. 486C. neoformans and Candida albicans do not stain with the Congo red stain, whereas Blastomyces does.7. and 8. Errors in diagnosis can be avoided if molecular techniques are used to identify the organism in paraffin-embedded tissue. Antibody probes are now commercially available. 483
The primary inoculation form shows less epidermal hyperplasia and a mixed dermal infiltrate containing numerous budding organisms. There are usually no giant cells or granulomas.
Infection with Coccidioides immitis is most frequently an acute self-limited pulmonary infection resulting from inhalation of dust-borne arthrospores.499. and 500. The disease is endemic in the southwest of the United States, Mexico, and parts of Central and South America.501. and 502. C. posadasii is a recently renamed subspecies of C. immitis. 503 In less than 1% of cases, but particularly in immunocompromised patients, 504 dissemination of the infection occurs. The skin may be involved in disseminated disease, the cutaneous manifestations taking the form of a verrucous plaque, usually on the face,505.506.507. and 508. or subcutaneous abscesses, 509 pustular lesions,509. and 510. or rarely papules and plaques.511. and 512. Disseminated lesions mimicking mycosis fungoides have been reported. 513 A florid exanthematous eruption may also occur. 503 Primary cutaneous coccidioidomycosis is extremely rare and follows inoculation of the organisms at sites of minor trauma,514.515. and 516. particularly in laboratory517 or agricultural workers. 518 Cases of primary cutaneous coccidioidomycosis were reviewed in 2003. 515 Rarely lymphangitic nodules develop, similar to those in sporotrichosis. Erythema nodosum occurs in up to 20% of patients with pulmonary infections, and erythema multiforme and a toxic erythema may also occur. 509 Hypercalcemia is a rare complication of systemic disease. 519
DNA hybridization probe tests are now available commercially for the detection of coccidioidomycosis.515. and 520.
The prognosis of primary cutaneous coccidioidomycosis is excellent. Most lesions heal spontaneously without treatment. 515 Disseminated disease is usually treated with an azole antifungal agent or amphotericin B. 515 Azoles are contraindicated in pregnancy. 500
Established lesions show non-caseating granulomas in the upper and mid dermis, with overlying pseudoepitheliomatous hyperplasia of the epidermis. 505 Thick-walled spherules of C. immitis, which usually range from 10 to 80 µm in diameter, are present within the granulomas, often in multinucleate giant cells. 506 Endospore (sporangiospore) formation is often seen in the largest spherules (sporangia). 506 The spherules can usually be seen without difficulty in hematoxylin and eosin-stained preparations. They are sometimes quite sparse. Mycelial structures have been reported in the lungs, but they are exceedingly rare in cutaneous lesions. They take the form of septate hyphae. 521
Early lesions and subcutaneous abscesses show numerous neutrophils, with a variable admixture of lymphocytes, histiocytes, and eosinophils. 518 Eosinophilic abscesses may form. 507 There are only occasional giant cells. Organisms are usually abundant in these lesions.
Paracoccidioidomycosis, also known as South American blastomycosis, is a systemic mycosis confined to Latin America. 523 There are endemic areas in rural Brazil, Argentina, Colombia, and Venezuela.524. and 525. It is caused by the dimorphic fungus Paracoccidioides brasiliensis. 526 The respiratory tract is the usual portal of entry, from where hematogenous dissemination to other parts of the body occurs. 527 Disseminated paracoccidioidomycosis with skin lesions has been reported in a patient with AIDS. 528 Transcutaneous (primary cutaneous) infection is less common.529. and 530. Oral and mucosal involvement is frequently present in paracoccidioidomycosis, but cutaneous lesions are less common. There are usually several crusted ulcers when the skin is involved.531. and 532. More widespread disease is sometimes present. 533 Over 90% of cases occur in males. 527
The azole antifungals, particularly itraconazole, have largely replaced amphotericin B for the treatment of skin lesions in the absence of significant systemic involvement, because of their less toxic side effects.510. and 532. Azoles are fungistatic rather than fungicidal and relapses may occur some months after the cessation of treatment. Terbinafine and trimethoprim-sulfamethoxazole have also been used.534. and 535. Efforts to develop a vaccine are in progress. 502
Cutaneous lesions often show pseudoepitheliomatous hyperplasia overlying an acute and chronic inflammatory cell infiltrate in the dermis. 536 Granulomas are usually present and there may be foci of suppuration. The characteristic feature is the presence of small and large budding yeasts measuring 5–60 µm in diameter. 536 The buds are distributed on the surface in such a way as to give a ‘steering wheel’ appearance. 537 The organisms often have a thick wall with a double-contour appearance. 534 They can be found in macrophages and foreign-body giant cells and lying free in the tissues. They may be overlooked in hematoxylin and eosin-stained sections and are best seen with the Grocott silver methenamine stain.
Like other granulomatous diseases of infective etiology, there is a histological spectrum reflecting the immune response to the organism. The hyperergic pole is characterized by compact epithelioid granulomas and many cells expressing IFN-γ, and the anergic pole represented by parasite-rich lesions and poorly organized granulomas, and large numbers of cells expressing IL-5 and IL-10.524. and 535.
Histoplasmosis results from infection with Histoplasma capsulatum, a dimorphic soil fungus which is endemic in parts of America, Africa and Asia. In America, it is found in some areas of south-eastern USA, and in southern Mexico. The infection is acquired by the inhalation of spores from soil contaminated by bird and bat excreta. 538 The lung is the most usual primary focus of involvement, except in the African form (see below), and in 99% of cases the pulmonary infection is self-limited and asymptomatic. 539 Immunosuppression, including AIDS,538.540.541.542.543.544.545.546.547.548. and 549. old age, and chronic disease states predispose to disseminated disease.550.551.552. and 553. Cutaneous lesions occur in 5% or less of these patients.539.551. and 554. This secondary cutaneous form presents as papules, 541 ulcerated nodules, cellulitis-like areas, vasculitic lesions, 538 pyoderma gangrenosum-like lesions, 555 tumor-like lesions, 556 acneiform lesions, 542 or, rarely, as an erythroderma.557. and 558. Erythema nodosum is an uncommon manifestation of histoplasmosis. 559 Oral involvement has also been reported, 560 particularly in patients with AIDS.561. and 562. Rarely a cutaneous lesion is the only manifestation. 563 This primary cutaneous form usually presents as a solitary self-limited ulcerated nodule at the site of fungal inoculation. 564 Genital histoplasmosis is a rare presentation of primary disease. 565 Disseminated cutaneous disease is also rare. 566 A cutaneous id-like reaction has been reported in patients undergoing treatment for pulmonary histoplasmosis. 567
The African form of histoplasmosis usually presents with cutaneous granulomas or with skin lesions secondary to underlying osteomyelitis.568. and 569. Disseminated disease can also occur. The causative organism, H. capsulatum var. duboisii, has much larger yeasts but is otherwise identical in laboratory characteristics to H. capsulatum. 568
The laboratory diagnosis of histoplasmosis can be made by serological testing, nested PCR assay, positive cultures, or histological visualization of organisms in affected tissue. 570 The histoplasmin skin test is no longer considered useful because a positive reaction does not distinguish a current infection from a past one, and negative reactions often occur in disseminated disease. 551
Treatment varies according to the severity of the disease and the immune status of the patient. 560 Oral itraconazole is the treatment of choice for patients who have mild or moderately severe symptoms, and for maintenance therapy. 560 Amphotericin B is used for more severe disease, and in patients with AIDS. 561
Usually there is a granulomatous infiltrate in the dermis, and sometimes the subcutis,571. and 572. with numerous parasitized macrophages containing small ovoid yeast-like organisms, measuring 2–3 × 3–5 µm in diameter. There is often a surrounding clear halo. Langhans giant cells, lymphocytes, and plasma cells are usually present, except in some acute disseminated cases in which parasitized macrophages predominate (Fig. 25.11). Extracellular organisms are sometimes found. Transepidermal elimination of macrophages has been reported. Healing lesions show progressive fibrosis. In patients with AIDS there may be only a sparse inflammatory cell infiltrate. Leukocytoclasis, dermal necrosis, and cutaneous nerve parasitosis may be present in these cases.538.546. and 573.
Histoplasmosis. (A) There are parasitized macrophages and scant lymphocytes. (H & E) (B) Numerous organisms can be seen in this silver preparation. (Grocott stain)
In the African form the organisms measure from 7 to 15 µm in diameter. 569 Suppuration is sometimes present, 568 but the characteristic tissue reaction is the formation of multinucleate giant cells of classic ‘foreign-body’ type, in the cytoplasm of which are usually 5–12 organisms.
The PAS stain often stains the cell wall of Histoplasma poorly. 7 The broad-spectrum stain, methenamine silver, is always positive. Some organisms are also acid fast. 7
Histoplasma must be distinguished from Penicillium, which also parasitizes macrophages. Whereas Histoplasma produces small surface buds, Penicillium divides by schizogony with the formation of septa within the organism. The use of an immunoperoxidase technique assists in making a specific diagnosis of histoplasmosis on paraffin-embedded material. 565
The dematiaceous (pigmented) fungi are a clinically important group. 575 They are worldwide in distribution, although they are particularly prevalent in tropical and subtropical areas. 576 They are found in soil and decaying vegetable matter. The brown pigment of dematiaceous fungi is a melanin, which may be highlighted in tissue sections by the various stains for melanin. 577 They are capable of producing clinical diseases which range from a mild superficial cutaneous infection to life-threatening visceral disease. 578 Infection usually results from direct inoculation of infected material into the skin, but inhalation of organisms into the lungs may be the origin in some cases of systemic infection.
Various classifications have been proposed for the infections produced by dematiaceous fungi, with a proliferation of nomenclatures.576.579. and 580. Some of the fungi have been reclassified several times in the past 20 years, resulting in taxonomic confusion. There are two clinicopathological groups, chromomycosis and phaeohyphomycosis, 580 which represent extremes of a continuum of infections. 581 Pigmented fungi may also produce mycetomas, which are tumefactive lesions with draining sinuses and the presence of grains in the tissue. 582 These cases are best considered with the mycetomas caused by other organisms (see p. 600). 580
Chromomycosis is characterized by localized cutaneous infection and the presence in the tissues of thick-walled septate bodies (sclerotic bodies, muriform cells). 583 Phaeohyphomycosis580. and 584. (phaeochromomycosis, 576 chromohyphomycosis585) is a collective term for a heterogeneous group of opportunistic infections that contain dematiaceous yeast-like cells and hyphae. These infections can be seen following direct implantation of infected material, or in immunosuppressed patients, in whom the portal of entry is not always evident.
Chromomycosis (chromoblastomycosis) is primarily a disease of the tropics and subtropics; it is rare in Europe and North America. It is caused by saprophytic, pigmented fungi commonly isolated from plant debris and soil. Accordingly, it is an occupational hazard in some rural workers.
Chromomycosis starts as a scaly papule, often following superficial trauma, which slowly expands into a verrucous nodule or plaque.586.587.588. and 589. In most series there has been a predilection for the lower legs, but in Australia the upper limbs are most often involved. 586 The face, breast, and toenails are rarely infected.590.591. and 592. It may present with longitudinal melanonychia. 593 Also rare is dissemination with the formation of generalized cutaneous lesions, 594 lymphangitic nodules, or hematogenous lesions.578. and 583. Immunosuppression may predispose an individual to these more aggressive lesions. 595 Mild lymphedema is often present in the affected limb. 596
The eight species that have been incriminated include Fonsecaea pedrosoi597. and 598. (Phialophora pedrosoi), Phialophora compacta (Fonsecaea compacta),599. and 600. Phialophora verrucosa, Cladosporium carrionii, 601Aureobasidium pullulans, 602 and rarely Rhinocladiella aquaspersa (Acrotheca aquaspersa). 578 Although it is a well-recognized cause of phaeohyphomycosis, Exophiala spinifera has been documented as a cause of chromomycosis in only several cases. 603 The eighth species to cause human disease, Chaetomium funicola, was reported in 2007 from West Panama, as a single case report. 604 It is an opportunistic fungus found in soil. Although sometimes cited as a cause, 576Wangiella dermatitidis (Phialophora dermatitidis) is now no longer considered to be involved. 605F. pedrosoi is the most commonly isolated organism, except in Australia, where C. carrionii is usually responsible.
Squamous cell carcinoma may develop in chronic lesions of chromomycosis; they may run an aggressive course.606. and 607. Malignant melanoma may also supervene. 608
The treatment of choice for small lesions is surgery; they may be misdiagnosed clinically as skin cancers and treated accordingly. Treatment of lesions by cryosurgery with liquid nitrogen, 609 or with the carbon dioxide laser, 610 are other treatment options. Itraconazole alone, or with cryotherapy, has been used. 596 It may also be used with flucytosine. Intermittent, pulse itraconazole is a further option. 611 Terbinafine and fluconazole are other treatment options. Amphotericin B is now used for systemic or disseminated cutaneous disease. 591 Topical heat applications can supplement other therapies.
The appearances are similar to sporotrichosis, with hyperkeratosis, pseudoepitheliomatous hyperplasia, and granulomas in the upper and mid dermis. The granulomas are mostly of tuberculoid type, although a few suppurative granulomas are usually present. Intraepidermal microabscesses are often present, but these are not as numerous as in sporotrichosis. There is a background infiltrate of chronic inflammatory cells, and sometimes a few eosinophils, in the upper dermis. Round, thick-walled, golden brown cells (sclerotic bodies, muriform cells, medlar bodies) 5–12 µm in diameter can be seen in giant cells and lying free in the intraepidermal microabscesses (Fig. 25.12). 612 These sclerotic bodies are thought to be an intermediate vegetative form, arrested between yeast and hyphal morphology. 580 They are usually seen readily in hematoxylin and eosin preparations, and particularly in sections stained with hematoxylin alone. Stains such as PAS and silver methenamine often obscure the natural pigmentation of the fungal cells, and this can result in misdiagnosis of the type of fungus. Ziehl–Neelsen (ZN) and Wade–Fite stains can also be used to demonstrate these bodies. 613 The fungal cells have a diversity of internal ultrastructure.614. and 615. Hyphae have been reported in the stratum corneum in an otherwise typical case of chromomycosis,616. and 617. and in the dermis and subcutaneous tissue of another case. 618
Chromomycosis. (A) There is marked pseudoepitheliomatous hyperplasia. (B) A brown spore is present in the cytoplasm of a macrophage. This granuloma is more typical. (C) High-power view of a septate body. (H & E)
The pseudoepitheliomatous hyperplasia is probably the mechanism by which the transepithelial elimination of the fungal bodies and inflammatory debris takes place. 619 There is progressive dermal fibrosis, and this is quite prominent in treated lesions. 620
A rare and unusual response is the effacement of the dermis by a spindle cell, a fibrohistiocytic response with some cells demonstrating nuclear atypia and scattered mitotic figures. 621 Pigmented spores can be seen in the lesional giant cells. The fibrous response is thought to result from the high levels of pyridinoline produced by the fungi. 621
In sporotrichosis, foci of suppuration and suppurative granulomas are more obvious than in chromomycosis. Furthermore, the absence of organisms on a single hematoxylin and eosin section favors sporotrichosis as septate bodies are almost invariably found in a single section of chromomycosis.
Antigens of F. pedrosoi have been detected by immunohistochemical techniques in lesional skin, chiefly in macrophages, but also in Langerhans cells and factor XIIIa+ dendrocytes. 622
The term ‘phaeohyphomycosis’ is used for a diverse group of dematiaceous fungal infections580. and 583. whose hallmark is the presence in tissues of pigmented hyphae. Four clinical categories are recognized: superficial (black piedra and tinea nigra), cutaneous or corneal, subcutaneous, and visceral (systemic). 580 Some overlap exists between the cutaneous and subcutaneous forms. Thirty-six different species of fungi were listed in one review article as being involved in the etiology of phaeohyphomycoses. 583 The most commonly isolated fungi in cutaneous and subcutaneous lesions are Exophiala jeanselmei (which some mycologists now consider to be identical with Phialophora gougerotii),578.580.623.624.625. and 626. and Wangiella dermatitidis. 580 These fungi may also be isolated in the systemic forms involving the brain or other viscera. Cutaneous and/or subcutaneous lesions have also resulted from infection with Exophiala spinifera,627. and 628. E. dermatitidis, 629E. oligosperma, 630Curvularia pallescens, 631C. lunata,632. and 633. Nattrassia magniferae, 632Fonsecaea spp.,632. and 634. Hormonema dematioides, 635Bipolaris spicifera,636. and 637.Phoma sp.,638. and 639. Geniculosporium sp., 640Colletotrichum spp., 641Aureobasidium pullulans, 642Cladophialophora bantiana,643.644.645. and 646. Cladosporium cladosporioides,647Veronaea botryosa,648. and 649. Wallemia sebi, 650 and Phialophora richardsiae. 651 Other fungi involved in systemic infection include Cladosporium trichoides576 and Exserohilum sp.578.636.652.653. and 654. Patients with systemic infections are usually immunosuppressed.633.636.652. and 655.
Cutaneous lesions may be nodular, cystic, or verrucous. 656 They are usually solitary. Some patients are immunocompromised;482.657.658. and 659. in others, the lesion follows a penetrating injury with implantation of a wood splinter or other vegetable matter.660. and 661. The most common lesion is a subcutaneous cyst on the distal parts of the extremities. 662
Treatment of cutaneous phaeohyphomycosis includes surgical excision, oral itraconazole, terbinafine, and amphotericin B for disseminated cases.645.662. and 663.
The characteristic lesion is a circumscribed cyst or chronic abscess situated in the subcutis or lower dermis (Fig. 25.13). 667 The wall is composed of dense fibrous tissue with a chronic granulomatous reaction adjacent to the cavity. The wall also contains chronic inflammatory cells and scattered giant cells. There is a central cystic space containing necrotic debris with some admixed neutrophils. A wood splinter or similar foreign body is sometimes present. Brown filamentous hyphae and yeast-like structures may be present in the wall, in giant cells, or in the debris. Sometimes the fungal elements can be seen in relation to the implanted foreign material.668. and 669.
Phaeohyphomycosis. (A) A small wood splinter is present in the ‘cyst’. (H & E) (B) The fungal elements are present in the inflammatory lining of the cavity. (PAS–tartrazine)
Sporotrichosis is an uncommon fungal infection of worldwide distribution, caused by the dimorphic fungus Sporothrix schenckii. 671 It is endemic in Mexico and Central America, where infection rates in rural areas may approach one case per 1000 of the population.672. and 673. Infection usually results from percutaneous implantation of infected vegetable matter, particularly wood splinters, or from injury by rose thorns or contamination of even minor skin wounds by infected hay and sphagnum moss.674.675.676.677. and 678. Infection has also been acquired from animals,679.680. and 681. and in the laboratory. Most cases occur in adults, but children are sometimes affected.682. and 683.
There are three main clinical presentations of sporotrichosis: lymphangitic, localized (fixed), and disseminated. 684 The lymphangitic form (lymphocutaneous or sporotrichoid form) begins as a single nodule or ulcer and is followed by the development of subcutaneous nodules along the course of the local lymphatics. The initial and secondary nodules may ulcerate. This type accounts for 75% or more of all cases of sporotrichosis in many reports, but it is much less common in Australia and South Africa.675.685.686. and 687. The localized form (fixed form) presents as an ulcerated nodule or verrucous plaque which measures 1–5 cm or more in diameter.688. and 689. It has been suggested that this form is more likely to develop in patients previously sensitized to Sporothrix schenckii, but this does not explain why lesions in children,690.691.692. and 693. and those on the face,694. and 695. are often of this type. The subtype of the organism and the temperature sensitivity of the particular strain of organism involved have also been suggested as influencing the clinical type of lesion. 696 Over 30 subtypes of S. schenckii have been identified and the various subtypes show strong geographic specificity. 672 The upper limbs are the most common site of involvement in both types of sporotrichosis. Unusual sites of involvement include the penis, pubic region, and oral mucous membrane. 697 Spontaneous disappearance of lesions698 and exogenous second infections699 have also been documented.
Rarely, cutaneous lesions are erysipeloid700 or generalized, 701 the latter form usually occurring in association with visceral involvement.702.703. and 704. Such lesions may clinically mimic pyoderma gangrenosum.705.706. and 707. Visceral involvement (the disseminated form) may also occur without associated skin lesions. 708 The lungs, bones, joints, and meninges are favored sites of extracutaneous involvement.708.709.710. and 711. Disturbances in cell-mediated immunity (including AIDS),712. and 713. cancer, 709 and sarcoidosis714 have been present in some patients with disseminated sporotrichosis. 715 Co-infection with leishmaniasis has been reported. 716
Potassium iodide was the treatment of choice for many years. It is still used in some countries with endemic disease.682.697. and 717. Itraconazole has replaced it in some parts of the world. 673 Other azole, polyene, or allylamine antifungal agents have been used. 683 Heat therapy can be used alone or with antifungal agents. In Australia, where the localized form is the predominant type, excision of the lesion is often carried out, as the lesions are frequently misdiagnosed as squamous cell carcinomas.
In the localized form there is usually prominent pseudoepitheliomatous hyperplasia with some overlying hyperkeratosis and focal parakeratosis (Fig. 25.14). Several types of granulomas are found within the dermis, including tuberculoid, histiocytic, and suppurative granulomas. Multinucleate giant cells may be present at the periphery of the granulomas, and some of these are of foreign-body type. Intraepidermal microabscesses are not uncommon.
Sporotrichosis. This localized lesion is characterized by pseudoepitheliomatous hyperplasia of the epidermis and suppurative granulomas in the upper dermis. (H & E)
In the lymphangitic form the inflammatory nodules are situated in the lower dermis and there are usually no epidermal changes. There is often a more diffuse inflammatory infiltrate, and the granulomas are mostly of the suppurative type. Coalescence of abscesses may occur.
The traditional view has always been that fungal elements are infrequently demonstrated in human cases of sporotrichosis. In our study of 39 cases, only 2 of which were of lymphangitic type, fungal elements were found in all cases. This was achieved by examining multiple serial sections, some stained with hematoxylin and eosin and others with PAS. 718
The sporothrix may be present in the tissues as yeast-like forms 2–8 µm in diameter, or as elongated cells (‘cigar bodies’) 2–4 × 4–10 µm, or as hyphae. 719 Hyphae are particularly rare.719. and 720. A characteristic finding is the ‘sporothrix asteroid’: this is a yeast form (blastospore) surrounded by an intensely eosinophilic, hyaline material, ray-like processes of which extend for a short distance from the core (Fig. 25.15). The hyaline material stains faintly with PAS. This material represents deposits of immune complexes on the surface of the fungal cell. The central yeast stains with anti-Sporothrix antibodies but the surrounding eosinophilic, ray-like processes do not. 721 Asteroids are found only in the center of suppurative granulomas and suppurative foci. In our experience each such focus will contain an asteroid, if examined by serial sections. 718 Asteroids can also be found in fresh pus removed from lesional skin by compression. 722 Spores can usually be demonstrated using a silver methenamine or PAS stain. Prior digestion with malt diastase has been suggested as a method to enhance recognition of spores in PAS preparations. 723 Numerous spores are sometimes present, 724 particularly if the lesion has been injected with steroids because of a mistaken clinical diagnosis (Fig. 25.16). 725
Sporotrichosis. (A), (B) A Sporothrix asteroid is present in a suppurative granuloma. (H & E)
Sporotrichosis. Numerous fungal elements are present. This lesion was injected with corticosteroids because of a mistaken clinical diagnosis of granuloma annulare. (H & E)
Tinea nigra is a rare asymptomatic mycosis of the stratum corneum caused by a dematiaceous fungus, Phaeoannellomyces (Exophiala) werneckii.726. and 727. It presents as a slowly enlarging, brown to black macule, or large patch, involving the palms728.729.730.731. and 732. or palmar surfaces of the fingers, 733 and less commonly the plantar734. and 735. or lateral surfaces of the feet. 736 Lesions are sometimes bilateral. 734 Tinea nigra is more common in tropical areas. Children and adolescents may be involved. 732 Clinically, it may mimic various melanocytic lesions. 736
Topical antifungals from the imidazole family are the most effective treatment. 731
The stratum corneum may be slightly more compact than usual. Numerous brown hyphae are present in its superficial layers and are easily seen in hematoxylin and eosin-stained sections. Spores may also be shown by the PAS stain. There is usually no inflammatory reaction (Fig. 25.17).
Tinea nigra. Numerous brown hyphae are present in the superficial layers of the compact stratum corneum. (H & E)
Members of the genus Alternaria are plant pathogens. They are a rare cause of infection in humans, affecting both healthy737. and 738. and, more usually, immunodeficient individuals.739.740.741.742.743.744. and 745. The usual species, A. alternata, produces chronic crusted nodules, pustules, or ulcers localized to an exposed area such as the face, forearms, hands, and knees.746.747.748.749. and 750. Sometimes, large ulcerated plaques intermingled with pustules form.751. and 752. Subcutaneous nodules on the chest in association with A. dianthicola,739 and a nodule on the nasal septum caused by A. chartarum753 have been reported. A. chlamydospora, A. tenuissima, and A. infectoria are other extremely rare human pathogens.754.755. and 756. The rationale for including this group with the dematiaceous fungi, in the absence of pigmented tissue elements, is the formation of gray to black colonies on culture. Alternariosis is considered by some as a phaeohyphomycosis. 757
Treatment is usually with antifungal agents such as oral itraconazole or fluconazole, 758 but there is one report of thermotherapy being successful for a subcutaneous infection. 759
Usually there are non-caseating granulomas of tuberculoid, suppurative, or sarcoidal type, and chronic inflammation in the dermis, often with a few microabscesses. Sometimes the microabscesses are prominent. 761 Necrotizing folliculitis is sometimes present. 762 Epidermal involvement may accompany the dermal inflammation, or occur as the only manifestation. It is characterized by intraepidermal abscesses and often a thick scale crust containing neutrophils. Pseudoepitheliomatous hyperplasia is present in nearly half of the cases. Septate hyphae and spores can be seen in the dermis and epidermis. 763 PCR-based techniques can be used to confirm the diagnosis. 738
The organisms often show degenerative changes on electron microscopic examination. 764
On a strict etiological basis, only the eumycetic mycetomas (mycetomas caused by true fungi) should be included in this chapter. As will be seen from the discussions that follow, a similar tissue reaction can be produced by filamentous bacteria of the order Actinomycetales (actinomycetic mycetoma), and certain other bacteria (botryomycosis). 765 This is the rationale for the inclusion here of actinomycosis, nocardiosis, and botryomycosis.
Mycetoma is an uncommon chronic infective disease of the skin and subcutaneous tissues, characterized by the triad of tumefaction, draining sinuses, and the presence in the exudate of colonial grains.766.767.768. and 769. The sinuses do not develop until relatively late in the course of the disease, discharging grains which are aggregates of the causal organism embedded in a matrix substance.770. and 771. There are two main etiological groups of mycetoma: actinomycetic mycetomas, which are caused by aerobic filamentous bacteria of the order Actinomycetales, and eumycetic (maduromycotic) mycetomas caused by a number of species of true fungi.772.773. and 774. The therapy of these two groups is quite different. 775 Similar clinical lesions can be produced by traditional bacteria (botryomycosis), and rarely by dermatophytes.767.776.777. and 778. Dermatophyte-induced mycetoma must be distinguished from a kerion (see p. 584). The term pseudomycetoma is used for deep infections by dermatophytes in which draining sinuses are absent.34.35. and 779.
Mycetoma is predominantly a disease of tropical countries, particularly West Africa, parts of India, and Central and South America. 780 There are only sporadic reports of cases in the United States, 781 Canada, Europe (including the United Kingdom), 782 and Australia. 783 Different species predominate in different countries.780.781.782.783. and 784. For example, in Mexico, up to 85% of cases are caused by Nocardia brasiliensis, 785 while in Yemen, eumycetomas caused by Madurella mycetomatis predominate and lesions caused by Nocardia are uncommon786 Rural workers, particularly males, are most commonly infected. Over 70% of infections occur on the feet (Madura foot), with the hand the next most common site of involvement. Perianal lesions have also been reported. 787 Isolated cases of eumycetoma have been reported in recipients of organ transplants. 788
Repeated minor trauma or penetrating injury provides a portal of entry for the organism, which then produces a slowly progressive subcutaneous nodule after an incubation period of several weeks or months.780.789. and 790. Sometimes there is no history of trauma. 791 Sinuses develop after 6–12 months. Extension to involve the underlying fascia, muscle, and bone is common. Rarely there is lymphatic dissemination to regional lymph nodes. 771 No unequivocal cases of visceral dissemination have been reported. 792 Actinomycetic mycetomas often expand faster, are more invasive, and have more sinuses than eumycetic variants. 780 Helical computed tomography has been used to evaluate the invasion of actinomycetoma. 793
The grains discharged from the sinuses vary in size, color, and consistency, features that can be used for rapid provisional identification of the etiological agent. 780 Over 30 species have been identified as causes of mycetoma, and the grains of many of these have overlapping morphological features. Accordingly, culture is required for accurate identification of the causal agent.
The size of the grains varies from microscopic to 1–2 mm in diameter. Large grains are seen with madurellae (particularly Madurella mycetomatosis), and with Actinomadura madurae and A. pelletieri, whereas the granules of Nocardia brasiliensis, N. cavae, and N. asteroides are small. Discharging grains are not always seen. They were not seen in a mycetoma caused by Scytalidium dimidiatum, an extremely rare cause of this condition. 794
The colors of the grains of the most common species are shown in Table 25.1. Dark (black) grain mycetomas are found only among the eumycetic mycetomas. 795 The pigment is a melanoprotein or related substance. 796 The consistency of most grains is soft, but those of Streptomyces somaliensis and Madurella mycetomatis can be quite hard. 771Cladophialophora bantiana, usually associated with cerebral infections, has now been responsible for nine cutaneous cases; they were reviewed in 2005. 797 It has black grains.
The characteristic grains are found in the center of zones of suppuration and in suppurative granulomas in the subcutis (Fig. 25.18). Neutrophils sometimes invade the grains. Surrounding the areas of suppuration there may be a palisade of histiocytes, beyond which is a mixed inflammatory infiltrate and progressive fibrosis. A few multinucleate giant cells are usually present. An eosinophilic fringe, resembling the Splendore–Hoeppli phenomenon found around some parasites, is sometimes present around the grains.
(A) Mycetoma. (B) An irregularly shaped grain is present in the center of a zone of suppuration. (H & E)
Several reviews have discussed the morphology of the grains on light microscopy.771. and 780. Some of these features are highlighted in Table 25.2.799.800. and 801. It should be noted that the granules in pale-grain eumycetomas are not morphologically distinctive and there is overlap between the various species.
The large segmented mycelial filaments (2–4 µm in diameter, with club-shaped hyphal swellings and chlamydospores) which characterize the fungi that cause eumycetomas (Fig. 25.19) contrast with the Gram-positive thin filaments (1 µm or less in diameter) of the organisms that cause actinomycetomas.766. and 783.
Eumycetoma. The fungal elements comprising this grain are easily seen. (PAS stain)
Nocardiae are usually Gram-positive, partially acid-fast bacteria which are native to soil and decaying vegetable matter.802. and 803. The common pathogenic species are N. asteroides, 804N. brasiliensis,805. and 806. and N. caviae.807. and 808. N. farcinica, now acknowledged to be a species distinct from N. asteroides, can produce severe systemic infections with subcutaneous abscesses. Other species are rarely implicated.809.810.811.812. and 813. The majority of cases of nocardiosis are septicemic infections, usually of pulmonary origin, in immunocompromised patients.804.814.815. and 816. Cutaneous lesions develop in approximately 10% or more of hematogenous infections.817. and 818. Primary cutaneous involvement can be of three different types: mycetoma, lymphocutaneous infection, and superficial cutaneous infections, which may take the form of an abscess, an ulcer, or cellulitis.806.819.820.821.822.823.824. and 825. The lymphocutaneous (sporotrichoid, 826 chancriform) infection, 827 which includes a cervicofacial variant in children, 828 resembles sporotrichosis in having subcutaneous nodules along the course of the superficial lymphatics.829.830.831. and 832. A history of trauma is often present in primary cutaneous lesions.804.830.833.834.835.836. and 837.
Whereas N. asteroides is responsible for most pulmonary, cerebral, and septicemic infections, N. brasiliensis is the most common nocardial pathogen in the skin.802.838.839. and 840. There have been many exceptions to this generalization.822.841.842.843.844. and 845. Both species of Nocardia have been isolated from a mycetoma on the forehead, 846 and both N. asteroides and Sporothrix schenkii have been isolated from a mycetoma of the forefoot. 847 A concurrent double infection with E. spinifera and N. asteroides, the latter producing lymphocutaneous nocardiosis, has been reported. 848
The treatment of choice for Nocardia is trimethoprim-sulfamethoxazole.787.815. and 849. Amoxicillin-clavulanate and dapsone were successful in two individual cases.785. and 811. Sometimes an intravenous cocktail of antibiotics is required including amikacin and imipenem.812. and 813.
There is usually a dense infiltrate of neutrophils in the deep dermis and subcutis, with frank abscess formation. 850 In chronic lesions there is a thin fibrous capsule and many chronic inflammatory cells. Necrosis, hemorrhage, and ulceration may all be present at times. 842
The organisms are not readily visible in hematoxylin and eosin-stained sections. Special stains show them to be fine, branched filaments that are Gram positive, usually weakly acid fast, and which stain with the silver methenamine method. 851 Colonial grains are uncommon in cutaneous lesions other than mycetomas. 852
Actinomycosis is a chronic, sometimes fatal, infection that may involve any part of the body.853.854.855.856. and 857. Its cutaneous manifestations include fluctuant swellings, which may progress to draining sinuses in the cervicofacial, thoracic858 or abdominal region. 859 In the latter site there is often underlying visceral involvement, or a history of previous surgery in the region. Perineal and perianal infections may also occur.787. and 860. Surgical procedures at other sites may result in primary cutaneous lesions, particularly in the presence of diabetes mellitus or immunosuppression.849. and 861. Multiple subcutaneous abscesses may result from hematogenous dissemination of a visceral infection. 862
The causal organism is Actinomyces israelii, a filamentous bacterium which is isolated with difficulty by anerobic culture. It forms tiny, soft granules that may be detected in the pus (‘sulfur granules’). A. israelii is a normal inhabitant of the oral cavity. Similar lesions can be caused by other actinomycetes, including species of Streptomyces and Actinomadura.863. and 864.
There are few reports in the dermatological literature on the treatment of actinomycosis. One case was successfully treated with a combination of amoxicillin and minocycline. 861 Long-term, high-dose penicillin is the treatment of choice. 857
The usual cutaneous lesion is a subcutaneous abscess. There are often several locules, which are separated by areas of granulation tissue in which foamy macrophages are present. 853 There is an outer zone of granulation and fibrous tissue at the periphery of the abscess cavities, and this contains lymphocytes, plasma cells, and some macrophages.
One or more colonial granules are present in the pus. These average about 300 µm in diameter, but may range up to 1–2 mm. 853 At the periphery of the granules, club-shaped bodies radiate in parallel fashion from the border. The clubs and matrix of the granules are Gram negative. The granules are composed of numerous slender beaded filaments that tend to be crowded at the periphery of the granules. 853 They are Gram positive but not acid fast. 865 They are usually PAS positive and stain gray or black with the silver methenamine stain. Free organisms are infrequently seen.
Botryomycosis (bacterial pseudomycosis) is an uncommon chronic bacterial infection of the skin or viscera in which small whitish granules composed of the causal bacteria are present in areas of suppuration.866.867. and 868. In the skin they are often discharged through draining sinuses. As the lesions closely mimic clinically and histologically those of mycetoma, botryomycosis is included in this chapter. 869
The cutaneous lesion is usually a large swollen tumor or plaque with nodular areas or ulcers, and discharging sinuses.870.871. and 872. The hands, feet and head, and the inguinal and gluteal regions are most commonly involved.873. and 874. Multiple cutaneous lesions in different parts of the body have been described.875. and 876. Botryomycosis has sometimes been reported in patients with diabetes, 876 or with various abnormalities of the immune system, 873 including AIDS.875.877.878. and 879. It has been documented in a patient with extensive follicular mucinosis, 880 at the site of localized corticosteroid injections, 881 and in association with adamantinoma of the tibia. 882
Gram-positive organisms, particularly Staphylococcus aureus, are usually involved, but Gram-negative organisms, including Actinobacillus lignieresi and Pseudomonas aeruginosa, 883 have also been incriminated.866. and 884. A mixed growth of organisms is rarely present.
Antibiotics are used to treat botryomycosis, the agent used depending on the sensitivity of the causative bacteria, and the immune status of the host. 885
The lesion resembles mycetoma, with a small granule present in the center of a suppurative zone. The granules are basophilic, usually with a surrounding eosinophilic zone which is PAS positive. 866 The bacteria can be identified by a Gram stain. In contrast to mycetoma, there are no filaments present. 869 Transepidermal elimination of the granules has been reported. 886
There is an increasing tendency to use the broader term ‘zygomycoses’, which covers infections caused by all fungi belonging to the class Zygomycetes,888.889.890. and 891. rather than the older term ‘mucormycosis (phycomycosis)’, which is restricted to infections caused by various species in one family of the order Mucorales. However, as the infections caused by fungi within the order Entomophthorales, which is the other major group of Zygomycetes, are clinically quite different from mucormycosis, the two groups of infections will be considered separately. 892 The zygomycoses commonly affect immunocompromised individuals. 893
Mucormycosis refers to opportunistic infections by fungi within the family Mucoraceae, one of the many families in the order Mucorales. 894 There are three important genera responsible for human infections: Rhizopus, Mucor, and Absidia. These fungi are widespread in nature, particularly in soil and decaying vegetable matter. 894Rhizopus oryzae is the predominant pathogen in this group, but many different species in these three genera have been implicated in human infections.895. and 896. A fourth genus in the order Mucorales, Apophysomyces is only a rare cause of cutaneous disease. 897 A fifth, Rhizomucor, is equally rare. 898 Two more genera, Saksenaea and Cunninghamella, complete this family. 899 Several clinical categories of mucormycosis have been delineated: rhinocerebral, pulmonary, disseminated (hematogenous), gastrointestinal, and cutaneous.900. and 901. The cutaneous lesions may be further divided into primary and secondary types.
Primary cutaneous mucormycosis is rare and usually develops in diabetics, in patients with thermal burns, and sometimes in those who are immunocompromised.889.896.902.903.904. and 905. Toxic epidermal necrolysis has been complicated by Mucor infection. 906 Lesions resulting from contaminated adhesive dressings were reported in the past.907. and 908. Visceral dissemination may follow a primary lesion in the skin.909.910.911. and 912.
Secondary cutaneous mucormycosis results from hematogenous seeding from a lesion elsewhere in the body. 913 Such patients may have underlying diabetes, leukemia, lymphoma, neutropenia, or be immunocompromised in some other way.899.913. and 914. Premature infants and renal transplant recipients may be at risk.915. and 916.
Cutaneous mucormycosis may present as a tender indurated large plaque with a dusky center, as an area of necrotizing cellulitis, 892 as an area of necrosis in a thermal burn, or as a lesion resembling ecthyma gangrenosum.900. and 917. It may also mimic pyoderma gangrenosum. 898 In one instance a large ulcer developed in a tattoo; 888 another report has documented involvement of the skin around the site of an intravenous catheter insertion. 918 A bull’s-eye infarct developed in another patient at the site of an arterial line. 919 Infection has also occurred at the site of an insect bite. 920 Penile lesions due to Rhizopus oryzae and, in another instance, to Absidia corymbifera have been reported.921. and 922.
Amphotericin B, in both the conventional and liposomal forms, has been used to treat this disease.895. and 919. Itraconazole and voriconazole have also been used. The new triazole antifungal agents, such as posaconazole, have shown greater efficacy and less toxicity than amphotericin B and may become the treatment of choice. 901
The appearances are quite variable. There may be suppuration or areas of necrosis. There may be a resemblance to superficial granulomatous pyoderma, although the presence of fungal elements assists in making a distinction. 924 Sometimes there is only a minimal inflammatory response. Hyphae often invade vessel walls, with subsequent thrombosis and infarction.913. and 917. Subcutaneous granulomas have been reported with some species of Absidia.
The hyphae are broad and usually non-septate. They branch at right angles, in contrast to Aspergillus, which usually branches at an acute angle. 892 Sometimes the hyphae appear collapsed and twisted. They are often clearly seen in hematoxylin and eosin-stained sections.
Subcutaneous phycomycosis is the established, yet somewhat unsatisfactory, designation for infections caused by fungi of the order Entomophthorales. 925 Two genera, Basidiobolus and Conidiobolus (Entomophthora), are usually implicated in the infections, which typically are solitary and involve the subcutis of healthy individuals.926.927. and 928. Spontaneous resolution of the lesions sometimes occurs. Visceral spread has been reported in a renal transplant recipient. 929 These infections have been mainly reported from Africa and southeast Asia. 930Conidiobolus uncommonly causes subcutaneous disease. It has also been associated, rarely, with rhinofacial disease.931. and 932.
Potassium iodide or itraconazole can be used to treat basidiobolomycosis that is extensive or persistent. 933
Phycomycoses have also been reported in animals, particularly horses (‘swamp cancer’). 934 In addition to fungi from this order, aquatic fungi (particularly Pythium sp.) from a totally unrelated ‘class’ have been incriminated in equine phycomycosis. 935 They are mentioned here because the author has seen two cases of a periorbital cellulitis in humans who had contact with horses, which were probably the source of these equine fungi. 936
There is granulomatous inflammation in the dermis and subcutis, with scattered abscesses and sometimes areas of necrosis. 938 The most striking feature is the presence of smudgy eosinophilic material surrounding the hyphae.938. and 939. This resembles the Splendore–Hoeppli phenomenon seen in relation to certain metazoan parasites.940. and 941. Eosinophils are also present in the inflammatory infiltrate. 942
In the author’s human cases of equine phycomycosis, foci resembling the flame figures of eosinophilic cellulitis were present (Fig. 25.20). The fungi were not visualized on hematoxylin and eosin or PAS preparations, but only with the silver methenamine stain. 936
Equine phycomycosis in a human. (A) Multinucleate giant cells surround zones of necrosis. There are numerous eosinophils in the infiltrate. (H & E) (B) The hyphae are quite long on silver stain. They were not seen on the PAS stain. (Grocott stain)
The term ‘hyalohyphomycoses’ has been applied to a heterogeneous group of opportunistic infections in which the pathogenic fungi grow in tissue in the form of hyphal elements that are unpigmented, septate, and branched or unbranched.580. and 944. Dematiaceous fungi are excluded. Examples of hyalohyphomycoses include infections caused by Schizophyllum commune and species of Acremonium,945.946. and 947. Pseudallescheria,948.949. and 950. Paecilomyces,951.952.953.954.955.956. and 957. Fusarium, Penicillium, and Scedosporium.958.959.960.961.962.963.964. and 965. Infections caused by species of Aspergillus can also be accommodated in this group. One case of subcutaneous infection due to Cephalotheca foveolata, not previously thought to cause human infection, has been reported. 966 Predisposing immunological disturbances are often present in individuals with these infections; 963 immunodeficiency is not invariable.946.947. and 967. A contaminated skin lotion and a dog bite were the source of infection in two cases of Paecilomyces infection.952. and 968. Preterm neonates can also be involved. 969 Cutaneous lesions may take the form of disseminated nodules, 949 sporotrichoid spread, 964 crusted plaques, 968 and infection of surgical or trauma wounds. 950
Fusarium sp. can produce localized cutaneous lesions or disseminated disease. Systemic fusariosis mainly occurs in immunocompromised individuals with hematological malignancies, usually with associated neutropenia.970.971.972.973.974.975.976. and 977. It may also occur in patients infected with HIV, and occasionally in individuals with extensive burns. Trauma has also been implicated. 978 Disseminated fusariosis presents with sustained fever refractory to antibacterial and antifungal therapy, and with skin lesions in 60–70% of cases. In a series of 35 patients with cancer and Fusarium infection, reported from the M.D. Anderson Cancer Center in Houston, Texas, 20 had disseminated infection, 6 had primary localized skin infections, 4 had skin lesions associated with sinus infections, and 5 had onychomycosis. 979
Many different forms of skin lesions can be seen and most patients have a variety of forms. There may be red or gray macules, pustules, subcutaneous nodules, ecthyma gangrenosum-like lesions with a black eschar,980. and 981. target lesions, lupus vulgaris-like lesions, 982 vasculitic lesions, 983 and in one case a facial granuloma. 984 Most disseminated lesions occur on the extremities. 979
Onychomycosis can occur in both immunocompromised and immunocompetent individuals.979. and 985. The case reported on the arm of a healthy child986 was questioned in subsequent correspondence, because molecular identification was not carried out. 987 The genus Fusarium includes about 1000 species and variants. 987
The mortality rate is 50–80%, as it responds poorly to formulations of amphotericin B, and itraconazole. Voriconazole has had limited success. 988 Granulocyte colony-stimulating factor may be necessary to facilitate bone marrow recovery.
There is usually a heavy acute and chronic inflammatory cell infiltrate in the dermis and/or the subcutis. Granulomas are sometimes present. Dermal necrosis may be associated with mycelia invading blood vessels with subsequent thrombosis. 989 The hyphae of Fusarium are hyaline, septate, and branched at acute or right angles. They resemble Aspergillus.977. and 981.
In some southeast Asian countries, infection with the dimorphic mold Penicillium marneffei has increased in importance; it is an indicator organism of advanced HIV infection.990.991.992.993. and 994. The majority of cases occur in northern Thailand or in individuals who have migrated from that area. 995 In addition to infecting patients with AIDS, it also occurs in patients with Hodgkin’s disease, tuberculosis, and autoimmune diseases. Infection is assumed to result from inhalation of conidia.
The majority of cutaneous lesions are umbilicated papules, resembling molluscum contagiosum. 990 There may be central necrosis. Brown papules and indurated macules also occur. 996 The lesions are primarily on the upper half of the body, including the oral mucosa. Fever, weight loss, anemia, and lymphadenopathy are often present.
The fungus can readily be isolated from skin lesions and a PCR-based method for diagnosis has been developed. 996
Treatment with amphotericin B, followed with oral itraconazole has been used. Lifelong itraconazole may be necessary for AIDS patients to prevent recurrence of the disease. 990
There is a diffuse dermal infiltrate composed mainly of histiocytes and lymphocytes. Numerous round to oval, thin-walled yeast-like organisms are scattered in the tissue or within parasitized macrophages. 996 The organisms have clear cytoplasm and round nuclei; they measure up to 5 µm in diameter. They have some resemblance to Histoplasma but differ by the lack of surface budding, and by the presence of central division with the formation of septa within the organism.
Aspergillosis is an opportunistic infection second only to candidosis in frequency among patients with cancer. 997 It usually involves the lungs, and only rarely the skin.998. and 999. Cutaneous lesions are usually part of a systemic infection in immunocompromised patients, although rarely they may be the only manifestation (primary cutaneous aspergillosis).330.918.998.1000.1001.1002.1003.1004.1005.1006.1007. and 1008. Many patients with primary cutaneous aspergillosis have leukemia, and lesions have developed at the sites of intravenous cannulae or of the associated dressings.1009.1010.1011.1012.1013. and 1014. Preterm infants may also develop this form of the disease. 1015 There are one or more violaceous plaques or nodules that rapidly progress to necrotic ulcers with a black eschar.1010.1016.1017. and 1018. Plaques studded with pustules are sometimes seen.1004. and 1019. Onychomycosis may also occur. 1020Aspergillus flavus is the most common isolate;1009. and 1021. A. fumigatus, A. ustus, 1022 and A. niger have also been responsible.1010. and 1023. Rarely, burns or pyoderma gangrenosum are secondarily infected with Aspergillus species.1024. and 1025.
Treatment is usually with amphotericin B, although it is not always successful. Caspofungin, voriconazole, flucytosine, terbinafine, and itraconazole have all been used at different times. 1018
Depending on the host response, there can be a variety of changes ranging from well-developed granulomas1000 to areas of suppuration and abscess formation, 1011 or the presence of masses of fungi with a minimal mixed inflammatory cell response. 998 Hyphae may invade the walls of dermal blood vessels producing thrombosis and some associated necrosis. 1026Aspergillus species are found as septate hyphae that branch dichotomously. They are best shown by the silver methenamine stain.
Overlying pseudoepitheliomatous hyperplasia of the epidermis has been reported. 1027
Only two infections are of dermatopathological importance – keloidal blastomycosis and rhinosporidiosis. Both have a limited geographical distribution.
Keloidal blastomycosis (Lôbo’s disease, lacaziosis) is a rare, chronic fungal disease in which slowly growing keloid-like nodules or ulcerated verrucous plaques develop on exposed areas of the body.1028.1029.1030. and 1031. Lymph node involvement occurs in up to 10% of patients.1032. and 1033. The infection is caused by the fungus Loboa loboi, which is found almost exclusively in Central and South America. 1034 Cases from other countries have been reported rarely. 1035 The terms Lacazia loboi and Paracoccidioides loboi have been favored in some taxonomic classifications.990. and 1036. The disease also affects dolphins. 1037 Dolphin-to-human transmission has been reported. 1038 Squamous cell carcinoma is a rare complication in chronic lesions. 1039
Treatment with the usual antifungal agents has not been effective, although clofazimine has shown some effect. 1036 Cryotherapy and surgical excision have also been used.
There is an extensive granulomatous infiltrate in the dermis composed of histiocytes and giant cells of Langhans and foreign-body types, together with a few small collections of lymphocytes and plasma cells. There are numerous unstained fungal cells in hematoxylin and eosin-stained preparations, both free and in macrophages, giving a characteristic ‘sieve-like’ appearance (Fig. 25.21). They have a somewhat refractile wall and measure 6–12µm in diameter. Some budding, with the formation of short chains, may be present. The organisms are PAS positive; they do not stain with mucicarmine.
(A) Keloidal blastomycosis. (B) Numerous fungi with slightly refractile cell walls are present in the cytoplasm of macrophages and giant cells. (H & E)
Rhinosporidiosis is a chronic granulomatous infection caused by the hydrophilic agent Rhinosporidium seeberi.990. and 1041. Although traditionally regarded as a fungus, it is now considered a protistan parasite belonging to the class Mesomycetozoea. 990 Rhinosporidiosis usually presents as polypoid lesions of the nasal and pharyngeal mucosa. Cutaneous lesions are rare, even in India, Sri Lanka, and South America, where the causative agent, Rhinosporidium seeberi, is endemic.1042.1043.1044.1045. and 1046. Several cases have been reported from rural Georgia in the United States. 1047 The skin may be affected by contiguous spread from a mucosal lesion, by autoinoculation, and rarely through hematogenous dissemination.1048.1049. and 1050. The term ‘rhinosporidioma’ has been proposed for the solitary tumor-like nodule that occurs, rarely, on other parts of the body.1051.1052. and 1053.
The large spherical sporangia (100–400 µm in diameter), containing from hundreds to thousands of endospores, each measuring up to 7 µm in diameter, are characteristic (Fig. 25.22). The sporangia may be within gigantic foreign body giant cells. 1055 There is also a mixed inflammatory cell infiltrate, with the formation of some granulomas.
Rhinosporidiosis. Large spherical sporangia contain numerous endospores. (H & E)
Algal infections are exceedingly rare. In addition to protothecosis (see below), the intake of the blue-green alga Spirulina platensis in a food supplement has produced a presumptive eruption that exhibited features of both bullous pemphigoid and pemphigus foliaceus. 1056
Protothecosis is a rare infection caused by achlorophyllic alga-like organisms of the genus Prototheca.1057. and 1058. There are several species, but P. wickerhamii is most often found in humans.1059. and 1060. It can be cultured on Sabouraud’s medium, which is the usual medium used for the culture of fungi. 1061
Infection usually results from traumatic inoculation in an immunocompromised host.1062.1063.1064.1065.1066. and 1067. It may involve the skin and subcutaneous tissue.1068. and 1069. Infection of an olecranon bursa has been recorded. 1063 Rarely there is visceral dissemination. Cutaneous lesions are eczematous, 1070 pustular, 1071 herpetiform, 1072 or papules1073 and papulonodules which may coalesce, resulting in the formation of slowly progressive plaques.1063.1074. and 1075. Onychoprotothecosis can also occur. 1076
Treatments have included the use of amphotericin B, fluconazole, and itraconazole. Voriconazole has also been used in recent times. 1077
The usual picture is a chronic granulomatous reaction throughout the dermis, with a variable admixture of lymphocytes, plasma cells, and occasionally eosinophils and even neutrophils. 1078 In early lesions there are fewer multinucleate giant cells and the infiltrate shows greater localization to a perivascular and periappendageal position. There is focal necrosis in some lesions, particularly those with subcutaneous involvement. Epidermal changes are variable. 1062
Organisms (sporangia) are found in the cytoplasm of macrophages and multinucleate giant cells as thick-walled spherical bodies, often with a clear halo around them (Fig. 25.23). They may also be free in the dermis, but are difficult to see in hematoxylin and eosin-stained preparations. Prototheca wickerhamii, the species usually responsible for the infection in humans, measures from 3 to 11 µm in diameter. Many show internal septation, with endospore formation. Protothecal sporangia are much smaller than those of Coccidioides immitis. 1069 They stain with the PAS and silver methenamine stains. Specific identification can be made with a fluorescein-labeled monoclonal antibody.
Protothecosis. Sporangia are present in the cytoplasm of multinucleate giant cells. (PAS stain)
References
Introduction
1. McGinnis, MR, Dematiaceous fungi, In: (Editors: Lennette, EH; Balows, A; Hausler Jr, WJ; Shadomy, HJ) Manual of clinical microbiology 4th ed (1985) American Society for Microbiology, Washington DC, pp. 561–574.
2. Kimura, M; McGinnis, MR, Nomenclature for fungus infections, Int J Dermatol 37 (1998) 825–826.
3. Elewski, BE; Hazen, PG, The superficial mycoses and the dermatophytes, J Am Acad Dermatol 21 (1989) 655–673.
4. Lim, S-L; Lim, CS-H, New contrast stain for the rapid diagnosis of pityriasis versicolor, Arch Dermatol 144 (2008) 1058–1059.
5. Mann, JL, Autofluorescence of fungi: an aid to detection in tissue sections, Am J Clin Pathol 79 (1983) 587–590.
6. Monheit, JE; Cowan, DF; Moore, DG, Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy, Arch Pathol Lab Med 108 (1984) 616–618.
7. Veerappan, R; Miller, LE; Sosinski, C; Youngberg, GA, Narrow-spectrum staining pattern of Pityrosporum, J Cutan Pathol 33 (2006) 731–734.
8. Axelson, GK; Giorgadze, T; Youngberg, GA, Evulation of the use of Congo red staining in the differential diagnosis of Candida vs. various other yeast-form fungal organisms, J Cutan Pathol 35 (2008) 27–30.
9. Anthony, PP, A guide to the histological identification of fungi in tissues, J Clin Pathol 26 (1973) 828–831.
10. Graham, AR, Fungal autofluorescence with ultraviolet illumination, Am J Clin Pathol 79 (1983) 231–234.
11. Byrd, J; Mehregan, DR; Mehregan, DA, Use of anti-bacillus Calmette-Guérin antibodies as a screen for organisms in sporotrichoid infections, J Am Acad Dermatol 44 (2001) 261–264.
12. Moskowitz, LB; Ganjei, P; Ziegels-Weissman, J; et al., Immunohistologic identification of fungi in systemic and cutaneous mycoses, Arch Pathol Lab Med 110 (1986) 433–436.
13. Liu, D; Coloe, S; Baird, R; Pedersen, J, Molecular determination of dermatophyte fungi using the arbitrarily primed polymerase chain reaction, Br J Dermatol 137 (1997) 351–355.
14. Gräser, Y; El Fari, M; Presber, W; et al., Identification of common dermatophytes (Trichophyton, Microsporum, Epidermophyton) using polymerase chain reactions, Br J Dermatol 138 (1998) 576–582.
15. El Fari, M; Tietz, H-J; Presber, W; et al., Development of an oligonucleotide probe specific for Trichophyton rubrum, Br J Dermatol 141 (1999) 240–245.
16. Kac, G; Bougnoux, ME; Feuilhade de Chauvin, M; et al., Genetic diversity among Trichophyton mentagrophytes isolates using random amplified polymorphic DNA method, Br J Dermatol 140 (1999) 839–844.
17. Yang, C-Y; Lin, T-L; Tzung, T-Y; et al., Direct identification of dermatophyte DNA from clinical specimens by a nested polymerase chain reaction assay, Arch Dermatol 143 (2007) 799–800.
18. Arabatzis, M; Bruijnesteijn van Coppenraet, LES; Kuijper, EJ; et al., Diagnosis of common dermatophyte infections by a novel multiplex real-time polymerase chain reaction detection/identification scheme, Br J Dermatol 157 (2007) 681–689.
19. Shin, J-H; Sung, J-H; Park, S-J; et al., Species identification and strain differentiation of dermatophyte fungi using polymerase chain reaction amplification and restriction enzyme analysis, J Am Acad Dermatol 48 (2003) 857–865.
Superficial filamentous infections
20. Matsumoto, T; Ajello, L, Current taxonomic concepts pertaining to the dermatophytes and related fungi, Int J Dermatol 26 (1987) 491–499.
21. McGinnis, MR; Ajello, L; Schell, WA, Mycotic diseases. A proposed nomenclature, Int J Dermatol 24 (1985) 9–15.
22. Macura, AB, Dermatophyte infections, Int J Dermatol 32 (1993) 313–323.
23. Svejgaard, E, Epidemiology and clinical features of dermatomycoses and dermatophytoses, Acta Derm Venereol (Suppl) 121 (1986) 19–26.
24. Rinaldi, MG, Dermatophytosis: Epidemiological and microbiological update, J Am Acad Dermatol 43 (2000) S120–124.
25. Marks, R, Tinea incognito, Int J Dermatol 17 (1978) 301–302.
26. Grossman, ME; Pappert, AS; Garzon, MC; Silvers, DN, Invasive Trichophyton rubrum infection in the immunocompromised host: report of three cases, J Am Acad Dermatol 33 (1995) 315–318.
27. Svejgaard, E, Immunologic investigations of dermatophytoses and dermatophytosis, Semin Dermatol 4 (1985) 201–221.
28. Mayou, SC; Calderon, RA; Goodfellow, A; Hay, RJ, Deep (subcutaneous) dermatophyte infection presenting with unilateral lymphoedema, Clin Exp Dermatol 12 (1987) 385–388.
29. Sommer, S; Barton, RC; Wilkinson, SM; et al., Microbiological and molecular diagnosis of deep localized cutaneous infection with Trichophyton mentagrophytes, Br J Dermatol 141 (1999) 323–325.
30. Voisard, JJ; Weill, FX; Beylot-Barry, M; et al., Dermatophytic granuloma caused by Microsporum canis in a heart-lung recipient, Dermatology 198 (1999) 317–319.
31. Margolis, DJ; Weinberg, JM; Tangoren, IA; et al., Trichophytic granuloma of the vulva, Dermatology 197 (1998) 69–70.
32. Fernández-Torres, B; Mayayo, E; Boronat, J; Guarro, J, Subcutaneous infection by Microsporum gypseum, Br J Dermatol 146 (2002) 311–313.
33. Kobayashi, M; Ishida, E; Yasuda, H; et al., Tinea profunda cysticum caused by Trichophyton rubrum, J Am Acad Dermatol 54 (2006) S11–13.
34. Berg, JC; Hamacher, KL; Roberts, GD, Pseudomycetoma caused by Microsporum canis in an immunosuppressed patient: a case report and review of the literature, J Cutan Pathol 34 (2007) 431–434.
35. Petrov, I; Kempf, W; Stoilova, D; et al., Disseminated dermatophytic pseudomycetomas arising in an immunocompromised patient, Br J Dermatol 155 (2006) 628–630.
36. Tateishi, Y; Sato, H; Akiyama, M; et al., Severe generalized deep dermatophytosis due to Trichophyton rubrum (trichophytic granuloma) in a patient with atopic dermatitis, Arch Dermatol 140 (2004) 624–625.






