Chapter 48 Musculoskeletal changes secondary to thermal burns
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Information
Among trauma states the burn is alone in the tendency of its wound, even as it heals, to create musculoskeletal deformity. In addition, the protracted burn illness that accompanies severe burns may result in other skeletal change. Box 48.1 presents a classification of musculoskeletal changes secondary to burns; from that, the most commonly occurring and clinically significant alterations have been selected for discussion.
Changes confined to bone
Osteoporosis
Osteoporosis is the most frequently occurring post-burn change involving bone. Klein’s ongoing studies suggest that, among persons with serious burns, reduction of bone mass density is pervasive.2 Stated causes of osteoporosis in thermal burns are bed confinement, immobilization, hyperemia,3 reflex vasomotor phenomena,4 and adrenocortical hyperactivity.5 In Chapter 26 of this book, Klein reviews the effects of burn injury on bone metabolism.2,6 In this section only what is clinically apparent is discussed.
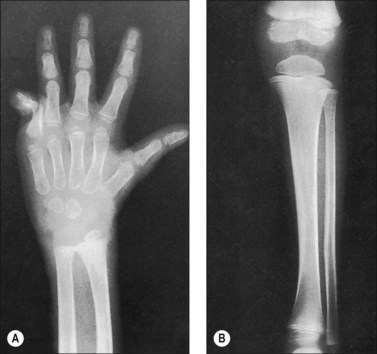
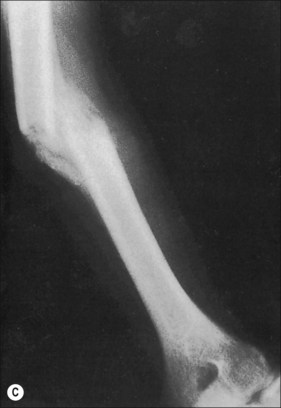
The more extensive the burn and the greater the number of complications, the longer the patient may be bed-confined and relatively immobile. The onset of osteoporosis is accelerated and its intensity more marked in the burn illness that features a hypermetabolic state. If a single extremity of an otherwise normal person is immobilized for a long period of time because of local trauma, as with a fracture, loss of bone density can be easily seen in a plain radiograph. So with burns isolated to the extremities, the bones of affected extremities become osteoporotic; and, in persons with generalized burns, the bones of deeply burned extremities may show more profound mineral loss than is observed in non-burned extremities or in the axial skeleton (Fig. 48.1). Van der Wiel et al.7 found in an X-ray absorptiometry study of 16 adults with fractures of one tibia that there was eventual loss of bone mineral density in the contralateral femur and in the lumbar spine, but to a lesser degree than in the ipsilateral femur. These findings, although not strictly analogous to those observed in burns, nevertheless point to the occurrence of generalized osteoporosis in other trauma states and the difference in loss of bone density relative to local factors. In fractures or in burns, impaired mobility and local hyperemia could account for this difference.
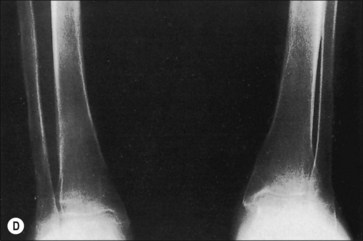
Another characteristic of the osteoporosis of burns that seems to set it apart is its persistence, not just until restoration of the anabolic state, but for months and years after the burn has healed (Fig. 48.2). This phenomenon may be most clearly observed in patients who have survived 90% burns, but Klein records less than normal bone among even moderately burned children as long as 17 months after injury.2 Muscle atrophy and/or the failure or inability of the person to return to the preburn level of physical activity may account in part for this protracted state of reduced bone mineralization.
Osteomyelitis
With open fractures at the base of a major soft tissue burn wound, bone infection is probably inevitable. Even so, the infection tends to remain at the fracture site and not to involve the rest of the bone, so that local debridement and stabilization of the soft tissue wound are all that are required for treatment. Dowling reported osteomyelitis of the tibia related to an open bimalleolar fracture in an extensively burned extremity.8 On the other hand, osteomyelitis developed in neither of the two open fractures reported separately by Choctaw9 and Wang.10 We treated three patients in whom open fractures of the femur complicated thigh burns. Each case required aggressive and repeated debridement. One fracture was treated in traction, while the other two were treated with external fixators. In one of the patients, who was admitted 8 months after acute burn, there was established osteomyelitis of the femur in relation to the exposed fracture. Osteomyelitis did not develop in either of the other patients; in the end, all three had sound femurs.
• the introduction or migration of organisms from the burn wound;
• linear pressure of the traction pin;
• excessive movement of the extremity leading to loosening of the pin;
Local low-grade infections usually resolve when pins are removed if the pin sites are vigorously curetted of granulation tissue. In one case in which a four-pin custom external fixator was used in the treatment of an open infection of the elbow, there resulted diffuse osteomyelitis of the humerus and radius. The infection was controlled with antibiotics and without surgery after the pins were removed. This case was included in Barret’s report of skeletal pinning in 41 severely burned children.11 In experience with the Ilizarov system for correction of skeletal deformity in burns, one patient developed a pin track infection of such severity as to require removal of the pin, curettage, and intravenous antibiotics for control of methicillin-resistant Staphylococcus.12
Fractures
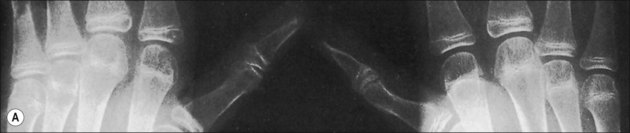
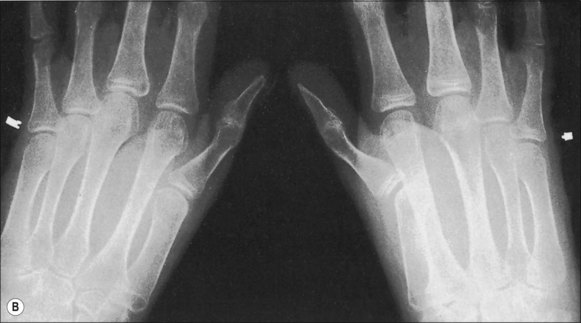
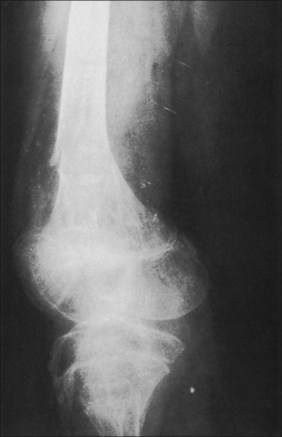
Pathological fractures were at one time common in burn management because of the practice of delayed excision of eschar and of keeping patients in bed until wounds were completely covered. During that time, fractures occurred because of bone collapse when patients first stood or walked or when stiff joints were manipulated (Fig. 48.3).13 The bones most commonly affected were the femur at its distal metaphysis and the tibia at its proximal one. The only treatment required was support of the extremity until the fracture consolidated, usually in 4–6 weeks. Children were more often affected than adults and the fractures usually compressed one cortex, producing an angular deformity that rapidly corrected with growth. Klein’s study2 strongly suggests that fractures occur more frequently in burned children than in a matched normal population even months after the acute burn. Now, however, in acute burn management the most frequently seen fractures are those occurring at the time of, or in association with, the burn injury. Falls or violent trauma account for many of the fractures, and the sites are those common to the causes, bearing no relation to the burn itself.
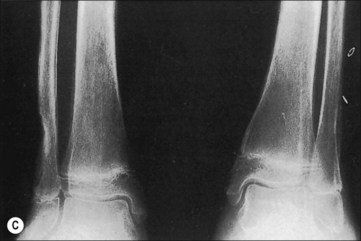
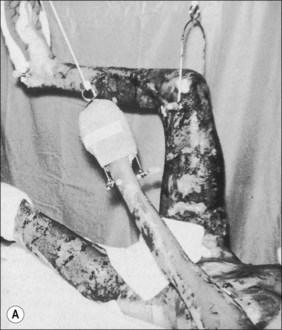
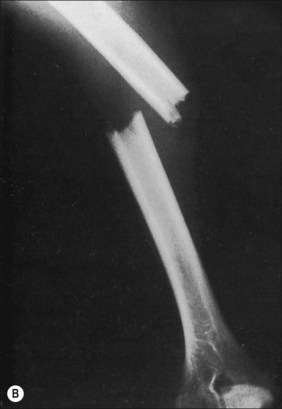
Although fractures complicate burn treatment and occasionally delay mobilization of patients, their management need not be complex. Fractures in extremities not burned can be treated by manipulative reduction and cast immobilization, by open reduction and fixation, with an external fixator, or with skeletal traction (Fig. 48.4). Fractures in extremities with first-degree or superficial second-degree burns can be managed in the same way. Deep second-degree and third-degree burns present a different problem only with respect to the early bacterial colonization of third-degree burns and the degradation of deep second-degree burns to full-thickness burns that will, in turn, become colonized. There is a precious window of time when fractures requiring open reduction and internal fixation can be definitively treated without increased risk for infecting the bone; however, fracture reduction and stabilization are so important in the functional management of a severely burned patient that the risk for bone infection should be acknowledged and shouldered at any post-burn stage. Skeletal traction can often be the management choice, particularly in children and adolescents, even if the treatment protocol requires that the patient be moved from bed for tubbing, dressing change, or additional surgery. The disadvantages of skeletal traction are the confinement to bed and the imposed relatively fixed position of the affected extremity. External fixators have made it possible to align and stabilize fractures in burned extremities without open operation. There is the added functional advantage of patient mobility. Brooker’s extensive favorable experience supports this concept.14 With both skeletal traction and external fixation, there is an added risk for bone infection because of the path from surface to bone provided by the pins. This is a risk worth taking, and it is minimized by scrupulous pin site care and by removal and replacement of any loosening pin. Frye and Luterman recognized and discussed the specific and continuous difficulties encountered in the management of fractures and burns.15
When casts are used for stabilization of fractures in burned extremities, the wound is made inaccessible and there is an abiding fear that the unattended wound will seriously degrade or at best not improve. Such fear may be well founded; however, Wang10 showed that a bivalved circular cast could be used effectively for an open comminuted fracture of a proximal tibia with overlying deep burns, and Choctaw9 reported successful use of a cast for immobilization of an open comminuted fracture after immediate post-burn grafting of the affected extremity. Common sense should dictate which fractures can be treated with circular or bivalved casts or with splints. If a reduced or moderately displaced but aligned fracture is so stable as to require external support only for maintenance of alignment, then cast or splint immobilization should be all that is needed. On the other hand, if a fracture because of instability, requires maintenance of reduction by three-point pressure or molding of the cast material, it will be better treated by other means.
Dowling8 reported osteomyelitis resulting from open bimalleolar fracture in an extremity with extensive deep burns. In neither of the cases reported by Wang and Choctaw did the bone become infected. There were also no infections among Saffle’s 42 fractures, nine of which were treated by open reduction and internal fixation.16 With two fractures of the femur, each of which was exposed at the base of a deep chronic burn, aggressive debridement of the wounds and the fracture ends was followed by treatment with skeletal traction in one and by external fixation with the Ilizarov system in the other. Both fractures healed without further complication.
Changes involving pericapsular structures
Heterotopic bone
Heterotopic bone formation is a rare but functionally important complication of thermal burns. The incidence in a general burn population is reported to be somewhere between 1% and 3%.1,17–22 In select populations, the incidence may be higher, as it will be if patients with periarticular calcification are included in the statistics. For example, Tepperman et al. reported a 35.3% incidence among patients referred to a tertiary care center for rehabilitation.23 Jackson made the observation that the incidence of heterotopic bone could be expected to be less in institutions that admit patients with minor burns.24 Munster’s25 radiographic survey of 88 adult and teenaged patients with 160 burned upper extremities yielded a 16% incidence of pericapsular calcification; the 23% incidence reported by Schiele et al.1 included both heterotopic ossification and calcification. In the early routine radiographic study reported by Evans,13 periarticular calcification that did not progress to heterotopic bone was excluded from the final calculation of an incidence of 2%. The 3.3% incidence recorded by Kolar and Vrabec26 included patients with pericapsular calcification. Even if heterotopic bone occurs infrequently in thermal burns, it remains that once it develops it often compromises joint motion and is difficult to treat. In addition, its pathogenesis is still incompletely understood; thus, protocols for prevention may in fact miss the mark.
Pathogenesis
The metabolic changes occurring after thermal burn are increased metabolic rate; protein catabolism; ureagenesis; fat mobilization; glycogenolysis; gluconeogenesis; elevated glucose flow; and eventual total body weight loss.27,28 There is an accompanying suppression of the immune system that favors wound infection, but at the same time favors survival of skin allografts. Infection, failure of skin graft take, or anything that delays closure of a burn wound will extend the altered metabolic state. Although it might be assumed that there occurs, along with the metabolic upheaval, an adverse change in connective tissue milieu, the exact nature of such a change is not known. It is also not known what the metabolic changes have to do with the development of heterotopic bone, but it is apparent that the burn disease is necessary to its formation. Other factors to be considered in the genesis of heterotopic bone are percentage of burn, location of burn, period of confinement, osteoporosis, superimposed trauma, and genetic predisposition.
Percentage of burn
Most reported cases of heterotopic bone have had a 20% or greater total body surface area burn; however, heterotopic bone has been found in patients with as little as 10% third-degree burn.13 Peterson et al.,20 Munster et al.,25 and Elledge et al.19 have reported affected patients with total body surface area burns of 8%, 14%, and 12%, respectively. In addition, with the now-extensive experience with salvage of patients with 80% or greater burns, it is clear that heterotopic bone occurs no more frequently among these patients than in the general burn population. Thus, the percentage of burn is not a determining factor.
Location of burn
By no means has all of the reported heterotopic bone occurred in joints with overlying burn. In their initial report, Evans and Smith described heterotopic bone occurring a distance from any third-degree burn involvement.29 Johnston noted that in one of his three patients, the skin overlying one affected joint was not even superficially burned.30 If degradation of connective tissue milieu in burns is a total body phenomenon, it follows that heterotopic bone formation need not be burn site dependent. Thus, the location of the burn cannot alone be a determining factor.
Period of confinement
Evans and Smith expressed belief that length of bed confinement was an important factor in the development of heterotopic bone.29 At the time of that report, patients with even moderate burns might be kept in bed for several weeks. The consequences of prolonged confinement were loss of active range of joint motion and bone demineralization; it was thought that each of these adverse changes might contribute to the formation of heterotopic bone. Thus, any maloccurrence that necessitated longer confinement could be a factor in the pathogenesis. Kolar implicated wound sepsis as an independent factor, along with the length of confinement.26 Other investigators have not addressed the period of confinement as specifically as Kolar, and there have been no comparative studies of groups of confined and non-confined patients. Thus, it may never be determined whether the current aggressive practice of early mobilization of patients will have an effect on the incidence of heterotopic bone.