Muscle Flaps and Their Blood Supply
Jamie P. Levine
FLAPS
A flap is a unit of tissue that is transferred from a donor to a recipient site with its blood supply. Numerous classification schemes exist. Flaps may be characterized by their component parts (e.g., cutaneous, musculocutaneous, and osteocutaneous), their special relationship to the defect (local, regional, distant, or free), the nature of the blood supply (random vs. axial), and finally by the movement that is required for the flap to fill the desired defect (e.g., advancement, pivot, transposition, and interpolation). This chapter will focus on the blood supply and classification systems for muscle flaps and their associated uses. The blood supply to the muscle is intimately involved with the overlying fascia and skin. In general, muscle flaps are “axial” pattern flaps, with a known blood vessel oriented longitudinally within the flap. These vessels will have perforating branches that then supply contiguous territories including the overlying skin. With the advancement of and enthusiasm for perforator flaps, the knowledge of the muscle blood supply has expanded into blood supply for territories of tissues (Chapter 4). These territories can be harvested as composite flaps incorporating all of the tissue layers or individual components can be taken separately based on the perforator blood supply.
HISTORY
Skin and subcutaneous tissue was initially elevated as “random” pattern flaps either from a site adjacent to the wound or from a distant site. Due to unpredictable circulation, these flaps often went on to partial or complete necrosis. This era of flap surgery required adherence to length and width ratios in hopes of maintaining adequate vascularity for flap survival. “Delay” techniques (Chapter 1) were also utilized to augment the vascular supply. The great advance in this area was the identification of specific vascular pedicles in consistent and reliable locations (dorsalis pedis, groin flap, etc.). Since these flaps could be elevated with a defined vascular pedicle, it became possible to transfer larger flaps.1 These early axial flaps were a vast improvement with regard to size and reliability, but because they remained pedicled, they were limited to specific topographic locations.
Next, the identification of muscle flaps as a source of tissue offered tremendous flexibility and more options for wound coverage and defect reconstruction.2 Muscles are available in almost all topographical areas. As the vascular anatomy to these muscles was elucidated, it became possible to detach the muscle origin, insertion, or both and to transfer the muscle to a new site while maintaining vascular perfusion. The decision of which muscle to utilize for a given defect takes into account multiple factors: the size and location of the defect, damage to regional tissues, and the presence of exposed vital structures. The ability to transfer muscles changed the way we are able to manage complex wounds of any variety.
With increasing interest in muscle circulation, the contribution of muscle flap circulation to the overlying skin was then recognized. This further advanced our ability to close complex, composite defects with improved function, cosmetic appearance, and donor site variety. Each superficial muscle provides vascular connections via musculocutaneous perforating vessels to the overlying skin. With the identification of vascular connections to the skin, it became possible to include a segment of skin with the muscle flap.3 Prior to identification of the muscle skin territory, which allows its design as a musculocutaneous flap, the muscle flap was inset into the wound and exposed portions were skin grafted for coverage. With a composite of muscle and its overlying skin, defect closure can be accomplished with muscle, subcutaneous tissue, and skin. The ability to take an island of skin can also increase the coverage area of a single flap if it is designed appropriately and also allows for improved postoperative monitoring as either a free or pedicled flap (Figure 5.1).
As understanding of cutaneous blood supply increased, fasciocutaneous flaps were also described.4 Finally, Ian Taylor was able, through ink injection analysis, to put the various concepts of skin circulation together in its most coherent form and defined the concept of the angiosome (Chapter 4). These studies helped to define the vascular territories of the body (with an average of over 300 cutaneous perforators)5 and provide a reliable guide to composite flap design based on cutaneous vascular anatomy. These concepts of vascular anatomy have opened a new era of flap transfer by defining perforator anatomy. The separation between pure fasciocutaneous and muscle flaps has also been erased. It was believed that the pedicle to fasciocutaneous flaps was always along the intermuscular septae. With the knowledge of perforator vessel dissection, fasciocutaneous flaps can be dissected from muscle-based vessels and perforators.
The next step in utilizing this knowledge of muscular vascular anatomy has been the creation of specially designed, chimeric flaps that can include segments of as much muscle, skin, and other tissues as needed for the reconstruction. This knowledge of vascular anatomy has also developed the concept of the free form perforator flap.6 These flaps can contain any tissue zone supplied by a regional vascular perforator and allows individualization of tissue thickness and texture to the specific needs of the defect.
BLOOD SUPPLY
Blood Supply: Random Flaps
Any flap requires an adequate blood supply after transfer to survive. “Random” cutaneous flaps are based on unnamed smaller vessels. It was observed historically that the ratio of flap length to width was a critical variable for flap survival. These restrictions limit their reliability for use with large defects. When utilized appropriately, however, random flaps can be reliable first choices for coverage of smaller defects throughout the body. The term “random” really means that the surgeon does not know for sure if there is enough longitudinally oriented (axial) vessels to keep the flap alive.
Blood Supply: Axial Flaps
In contrast to random pattern flaps, axial pattern flaps are based on a reliable, anatomically defined vascular territory that is oriented longitudinally within the flap and that extends beyond the base of the flap. Since the description of the first axial flap (the deltopectoral flap) nearly four decades ago, the knowledge of the body’s various cutaneous angiosomes and subsequent exploitation of axially based flaps has grown significantly.7 The advances in anatomic vascular knowledge have increased the type and the reliability of axial pattern flaps and have fostered the development of microsurgical free flap transfer. Because of their significantly greater reliability, axial flaps (flaps with known blood supply) are preferred for coverage of moderate to large defects.
The reliability and volume of tissue that can be placed into a defect is markedly greater than with any type of random pattern flap. Due to the axially oriented circulation, delay procedures are often not necessary even when mobilizing large tissue volumes in one procedure based on this direct circulation. The only limitation, when pedicled, is the limited topographic arc of rotation. These limitations have been essentially overcome by microvascular free tissue transfer techniques (Chapter 8) that are limited only by the availability of recipient blood vessels.
Blood Supply: Delay Phenomenon
In order to extend the somewhat restricted size of random flaps, surgeons rely on the delay phenomenon. This is most commonly achieved by interrupting a portion of the normal blood supply to the flap without transferring the flap from its native position. The associated sublethal ischemia results in (1) opening of “choke” vessels that are normally closed allowing blood flow into the ischemic region of the flap, (2) reorientation of the vessels within the flap to a more longitudinal pattern, and (3) sprouting of new vessels within the flap through angiogenesis, and perhaps via vasculogenesis.8
Vessels within the flap also respond to the stress of delay by increasing in caliber. Most surgeons find it prudent to delay a flap for at least 7 days to 3 weeks prior to final transfer, thereby permitting a maturation of the process of neovascularization.
Incorporating a planned delay can significantly improve the chances of complete survival of a large random pattern cutaneous flap,9 as in patients with an impaired microcirculation (e.g., smokers and diabetics). Furthermore, delay is always considered if a flap demonstrates signs of ischemia or venous congestion after elevation. In such cases the procedure is best performed in a staged manner, following a period of delay. Obviously, a planned surgical delay requires appropriate staging. In these cases intermediate coverage of critical structures may be required to bridge the gap between surgeries. If at all possible, the resection or exposure of any critical structures such as bone, tendon, nerve, or vessels should be delayed till the final coverage is ready. This is not always possible, especially in traumatic wounds. In these cases a delay procedure may not be possible and another flap is chosen.
Vascular delay can also be utilized to extend the size of axial flaps. By pre-incising the skin and subcutaneous tissue in a musculocutaneous or fasciocutaneous flap, or dividing the nondominant or codominant vascular pedicles, the tissue in a pedicled axial flap is maximized. An example is the delay of a pedicled transverse rectus abdominis myocutaneous (TRAM) flap. Since the inferior epigastric vessels are the primary vascular supply to the TRAM, division of this pedicle and attempts to transfer the flap based on the superior pedicle often lead to areas of ischemia in the skin beyond the primary skin territory (zone one). By ligating the deep inferior epigastric vessels with or without incising the skin paddle 1 to 2 weeks prior to breast reconstruction surgery, a much larger and more reliable skin paddle can be transferred.
PATTERNS OF MUSCLE CIRCULATION
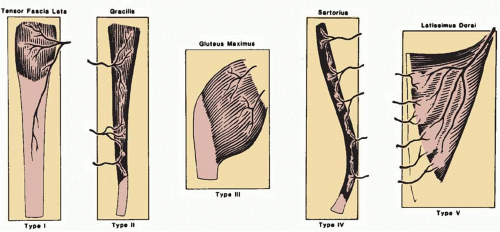
The most universally accepted system of muscle flap blood supply was developed by Mathes and Nahai. Every muscle, in part or as a whole, has the potential for use as a muscle flap. Muscle circulation is based on specific pedicles that enter the muscle between its origin and insertion and consist of an artery and single or paired venae comitantes.10 The position, number, and size of the vascular pedicles influence both the likelihood of flap survival and the flap design. The relative importance of each vascular pedicle to muscle circulation has been determined in cadavers by colored latex and barium injections, allowing evaluation of each vascular pedicle with regard to its length, diameter, location, and regional source. Subsequent use of the muscles as flaps has confirmed the relative importance of each pedicle to muscle survival and the potential for various flap modifications. If a pedicle to a muscle is critical to muscle survival based on its size and distribution to the internal vascular architecture of the muscle, it is specified as a dominant (where multiple pedicles are present) or major (where more than one pedicle is dominant) pedicle. Nondominant pedicles are labeled as minor pedicle(s). When a series of segmental smaller vessels are identified that may support muscle flap survival despite ligation of dominant or major pedicles, these minor pedicles are considered secondary pedicles. Variations in major and dominant pedicle anatomy are uncommon, although the location and number of minor pedicles is quite variable.
Five patterns of circulation to the muscle have been identified and are the basis of the classification system (Figure 5.2): Types I to V.
As noted above, Taylor et al. performed injection studies leading to the angiosome concept of the body. They noted that vessels frequently accompany nerves and described a classification system based on these observations.11 These studies revealed that many of the currently used flaps can be considered neurovascular flaps. Muscles were classified into four types according to their extrinsic and intrinsic neurovascular supplies. Type I muscles are supplied by a single unbranched nerve. In Type II muscles, the nerve branches before entering the muscle. Type III muscles receive multiple motor nerves from the same nerve trunk, and Type IV muscles are supplied from multiple nerve trunks. This system provides the clinical information necessary to divide muscles into functional neurovascular units for local and distant transfer.
MATHES AND NAHAI CLASSIFICATION
Type I: Single Vascular Pedicle
A single vascular pedicle enters the muscle and the muscle may be safely elevated on this pedicle.
Muscles identified with this pattern of circulation include the abductor digiti minimi (hand), abductor pollicis brevis,
anconeus, first dorsal interosseous, gastrocnemius, genioglossus, hyoglossus, longitudinalis linguae, styloglossus, tensor fascia lata, transversus and verticalis linguae, and vastus lateralis.
anconeus, first dorsal interosseous, gastrocnemius, genioglossus, hyoglossus, longitudinalis linguae, styloglossus, tensor fascia lata, transversus and verticalis linguae, and vastus lateralis.
Type II: Dominant Vascular Pedicle(s) and Minor Vascular Pedicle(s)
Use of a Type II flap generally requires division of part or all of the minor pedicles with preservation of the dominant pedicle. The muscle survives when elevated based on the dominant vascular pedicle. Muscles with a Type II vascular pattern include the following: the abductor digiti minimi (foot), abductor hallucis, brachioradialis, coracobrachioradialis, flexor carpi ulnaris, flexor digitorum brevis, gracilis, hamstring (biceps femoris), peroneus brevis, peroneus longus, platysma, rectus femoris, soleus, sternocleidomastoid, trapezius, triceps, and vastus medialis.
Type III: Dominant Pedicles
Type III muscles contain two large vascular pedicles, each of which may support the entire muscle. Muscles with a Type III vascular pattern include the following: gluteus maximus, intercostal, orbicularis oris, pectoralis minor, rectus abdominis, serratus, and temporalis.
Type IV: Segmental Vascular Pedicles
This group of muscles contains a series of segmental pedicles–generally of equal size–that enter the muscle along its course. Each segmental pedicle provides circulation to a portion (segment) of the muscle. Generally, division of two or more pedicles is feasible for transposition of a portion of the muscle as a flap. However, the muscle generally will not survive if an excessive number of the segmental pedicles are divided during flap elevation. Muscles with a Type IV vascular pattern include the following: the extensor digitorum longus, extensor hallucis longus, external oblique, flexor digitorum longus, flexor hallucis longus, sartorius, and tibialis anterior.
Type V: Dominant Vascular Pedicle and Secondary Segmental Vascular Pedicles
In this pattern of circulation, the muscle receives a large vascular pedicle that will reliably provide circulation to the muscle when it is elevated solely based on this particular vascular pedicle. However, the muscle has secondary vascular pedicles, which generally enter the muscle at its opposite end from the site of entry of the dominant vascular pedicle. These secondary pedicles will also support the muscle if the dominant vascular pedicle is divided. Thus, the muscle may be utilized as a flap based on either of the two sources of circulation. Muscles with a Type V pattern include the following: internal oblique, latissimus dorsi, pectoralis major.
ARC OF ROTATION
Each muscle and myocutaneous flap has a limited arc of rotation when transferred as a pedicle flap. The distance from the point where the pedicle enters the flap to the distal end of the flap defines the capability of that flap. A muscle flap that can be based on a dominant vascular pedicle can reach adjacent areas that fall within the radius created by the pedicle and the most distal portion of muscle that is supplied by that circulation. Generally, the muscle is released from either its origin or its insertion. The muscle is then mobilized on the major or dominant pedicle being utilized. In pedicle flap elevation, the pedicle is not usually skeletonized in order to avoid vascular injury and kinking. These rotational limitations should be incorporated into the surgical plan so that defect coverage will be maximized. With progressive mobilization of the pedicle, the arc of rotation of the flap can be increased. Release of the bony attachments overlying the point of entry of the vascular pedicle will also allow the muscle to be elevated as an island flap based only on its vascular pedicle with subsequent increase in its arc of rotation (Figure 5.3A-C).
Specific knowledge of anatomic landmarks including muscle insertion and origin and where the vascular pedicle enters the muscle will allow for better planning. A template can be made of the defect and then the arc of rotation of potential regional muscles can be plotted. Certain defects require two or more regional flaps, but knowledge of the muscular anatomy will allow reliable planning Muscle flap elevation based on the dominant pedicle is designated as the standard flap. If a muscle flap is elevated on its secondary pedicle, which requires division of the dominant pedicle, the flap is classified as a “reverse” flap. An example of this is a pectoralis muscle flap that is normally elevated on its dominant axial pedicle, the thoracoacromial vessels. The flap can also be raised as a turnover flap based on the secondary vessels from the internal mammary circulation, to cover a midline sternal defect.
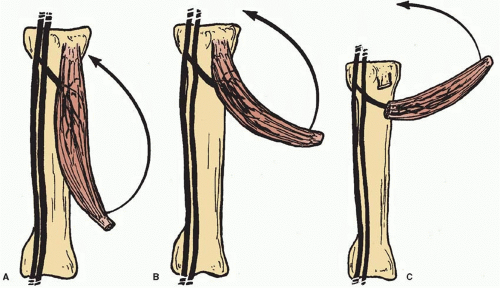 FIGURE 5.3. Arc of rotation. A. Arc of rotation with flap elevation to point of entrance of vascular pedicle to flap. B. Extended arc of rotation based on flap elevation with dissection of pedicle to regional source. C. Extended arc of rotation based on flap elevation with pedicle dissection and release of proximal fascia and/or muscle origin or insertion. (From Mathes SJ, Nahai F. Reconstructive Surgery Principles, Anatomy and Technique. Vol 1. New York, NY: Churchill Livingstone; 1997:115, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|