Fig. 12.1
ADSC spheroid with Hoechst staining (blue) of cell nuclei. Source: Kapur SK et al. “Human adipose stem cells maintain proliferative synthetic and multipotential propertion when suspension cultured as self-assembling spherads”. Bio fabrication 4(2) page 4, 2012 © IOP Publishing. Reproduced by permission of IOP Publising. All rights reserved
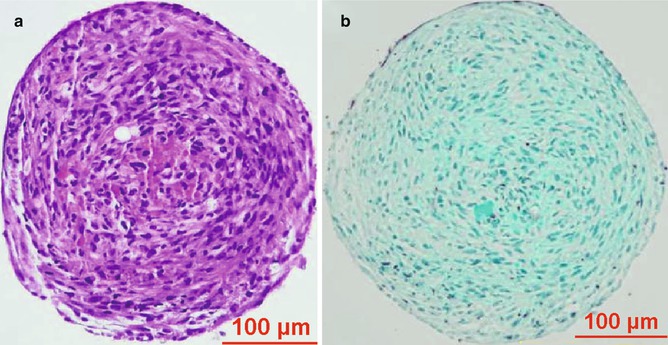
Fig. 12.2
Stained ADSC spheroid section containing abundant extracellular matrix. (a) H&E stain. (b) Trichrome stain. Source: Kapur SK et al. “Human adipose stem cells maintain proliferative synthetic and multipotential propertion when suspension cultured as self-assembling spherads”. Bio fabrication 4(2) page 4, 2012 © IOP Publishing. Reproduced by permission of IOP Publising. All rights reserved
Spheroids show predictable growth in size when cultured in growth factor-rich media and media containing human serum. They decrease in size when grown in spartan media conditions [16]. The maximal size that a spheroid can grow to may be limited by the distance that nutrients have to diffuse to reach cells near the core. This has been predicted by earlier studies, which describe the development of a necrotic core when spheroids grow to beyond 400 um in diameter [35]. The higher levels of angiogenic factors such as VEGF and HGF secreted by spheroids may be indicative of ischemic conditions at the core. In high growth factor media, these multicellular aggregates develop topographic polarity (Figs. 12.3 and 12.4). BRDU staining in these studies has shown spheroids to contain distinct dividing and nondividing cell populations, which could potentially contribute to the topographic polarity. The phenotypic differences between cells that make up these two populations are unknown at this time. Definitive causes of this polarization and its relationship to the media conditions have not yet been published [16].
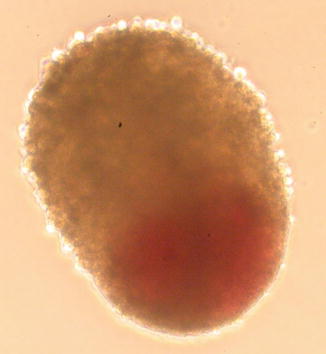
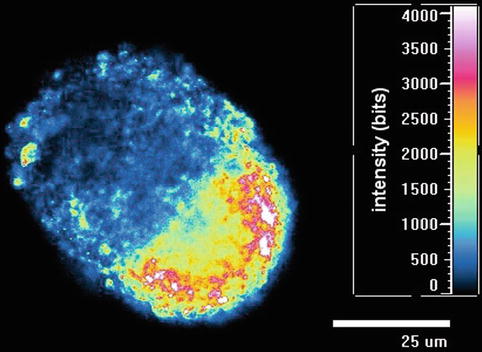

Fig. 12.3
5,000 cell ADSC spheroid showing topographic polarity when placed in high growth factor media

Fig. 12.4
5,000 cell ADSC spheroid showing topographic polarity when placed in high growth factor media with fluorescence intensity scale
12.6 Preservation of Stemness
ADSCs have proven to be useful multipotent cells because of their relative abundance, high proliferative capacity, and their resilience to remain viable and competent for long periods of time in culture. Under the correct media conditions, these cells can not only differentiate into cells specific to a mesodermal lineage but can also transdifferentiate into cells of ectodermal or endodermal lineages. The spheroid architecture has been shown to augment each of these attributes. ADSCs in spheroid architecture can remain competent even after 6 months in media and for about 1 month in no-serum/no-growth factor media conditions [16]. These spheroids when placed on culture plates give rise to monolayers of ADSCs, which are in turn competent to replicate and differentiate based on environmental cues. The spheroid-derived ADSCs initially have a low activity index and give rise to fewer colony-forming units compared to ADSCs grown as monolayers. The activity, however, quickly ramps up, and by day 14 of culture, ADSC spheroids have a significantly higher activity index and higher CFU-forming capacity than monolayer grown ADSCs [29]. Certain studies have shown that only 17 % of cells from dissociated spheroids can form CFUs [17]. The spheroid architecture preserves “stemness attributes” of ADSCs and may allow them to move into a more dedifferentiated state. As discussed earlier, when ADSCs are repeatedly cultured, they express more stromal cell markers and fewer stemness markers. When cultured as three-dimensional spheroids, ADSCs show a relative decrease in stromal cell marker expression and a relative increase in stemness marker expression. Repeated dissociation and reformation of spheroids, however, leads to a decrease in stemness marker (CD34) expression. PCR analysis of ADSC spheroids shows a three- to sevenfold higher expression of stemness genes such as Sox-2, Oct-4, and Nanog compared to monolayer-cultured ADSCs [29].
12.7 Improved Differentiation Capacity of Spheroids
In addition to providing a niche-like environment that preserves “stemness,” 3D architecture makes ADSCs more responsive to environmental cues. This is apparent in the improved differentiation capacity of ADSC spheroids when placed in inductive media. When ADSC spheroids are placed in adipogenic media (DMEM/F12, 0.5 mM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM insulin, 200 μM indomethacin, 10 % FBS, and 1 % ABAM), they begin to differentiate into adipocytes. This is confirmed by the increased expression of adipogenic markers LPL and PPARG. Positive oil red O staining indicates the presence of neutral lipids commonly seen in mature adipocytes. ADSC spheroids in osteoinductive media (DMEM/F12, 0.1 μM dexamethasone, 50 μM ascorbate) show increased expression of the osteogenic marker Runx2 compared to their ADSC monolayer counterparts. The spheroid cultures also show the presence of calcified matrix indicated by positive alizarin red staining. ADSC spheroids similarly show increased expression of chondrogenic markers collagen II and aggrecan compared to ADSC monolayers when placed in chondrogenic media (DMEM/F12, 6.25 μg mL − 1 insulin, 10 ng mL − 1 TGFB1, 50 nM ascorbate-2- phosphate, 1 % ABAM, and 1 % FBS) [16]. ADSCs are able to transdifferentiate into cells of endodermal and ectodermal lineages. In the presence of media containing HGF, FGF 1, and FGF4, ADSCs give rise to functional hepatocyte-like cells that are able to store glycogen, produce urea, and integrate into hepatocyte tissue architecture [36–38]. Media containing nicotinamide, activin-A, exendin-4, HGF, and petagastrin produce cells that behave like pancreas cells and are capable of insulin, somatostatin, and glucagon secretion [39]. ADSCs in spheroid architecture have been successfully shown to differentiate into hepatocytes and neural tissue and appropriately upregulate gene transcripts for nestin and albumin, respectively [29].
12.8 Synergistic Signaling Within Spheroid Architecture
An integral part of spheroid architecture is the presence of self-generated extracellular matrix. Microarray analysis of ADSC spheroids compared to ADSC monolayers has shown that ADSCs in spheroid architecture have a multifold increase in expression of genes related to extracellular matrix development and remodeling. ECM-related genes that are significantly upregulated include IGF-1, IGFBP-5, periostin, TGF-ß1, TGF-ß2, syndecan 1, syndecan 2, biglycan, collagen VIII A2, collagen XIV A1, collagen XV A1, collagen XVIII A1, collagen type VI A3, decorin, elastin, fibronectin 1, laminin A1, laminin ß1, MMP-1, MMP-2, MMP-8, MMP-9, MMP-14, TIMP-1, TIMP-2, TIMP-3, and tenascin C. Many of these proteins synergistically interact to amplify their effect. For instance, the ECM protein decorin complexes with TGF-ß and IGF-1 to increase their local concentration and amplifies their effect on wound repair [40]. Similarly, the provisional matrix protein, fibronectin, forms complexes with HGF to increase the rate of endothelial migration to wounds [41]. These synergistic interactions can explain the success of ADSC spheroid formulation in wound repair. ADSC spheroids have been reported to increase the closure rate of wounds in diabetic rodents to match those of nondiabetic controls. These benefits are not seen with non-3D formulations of ADSCs [20].
12.9 Hypoxia Resistance of Spheroids
In addition to augmenting the effect of the ADSC secretome, the extracellular matrix helps keep transplanted cells alive. The native extracellular matrix of monolayer ADSC cultures is generally digested by enzymes when ADSCs are dissociated prior to transplantation. Cells that lack cell–matrix interactions may perish in the transplanted environment. There is a consequent decrease in the number of transplanted ADSCs and reduction in their clinical potential. Spheroid culture provides ADSCs with abundant self-generated extracellular matrix, which is transplanted as part of the spheroid. Human nuclear antigen staining assays show that transplanted ADSCs in spheroid geometries persist in tissue for longer periods than monolayer ADSCs. The spheroid architecture also helps precondition ADSCs to better withstand hostile hypoxic environments. These ADSCs show a higher level of antiapoptotic proteins. They upregulate SCF-1 and HIF-1A, which in turn increase the secretion of proangiogenic cytokines, VEGF, HGF, and FGF-2. Cells of the host environment consequently show increased expression of adhesion molecules ICAM-1, VCAM-1, and PECAM-1 [42]. Under these hypoxic conditions, ADSCs within spheroids transdifferentiate and express endothelial cell markers (CD31, CD34, CD144). The spheroids also begin to show the presence of perfusion competent vessels [43].
12.10 Immunomodulatory Roles of Spheroids
ADSCs express various inflammatory markers such as IL-6, IL-8, IL-11, and TNF-alpha [22]. Similar to other mesenchymal stem cells (MSCs), ADSCs can play an immunomodulatory role. This has prompted their use in alleviating steroid-resistant graft versus host disease after hematopoietic stem cell transplantation. ADSCs have also been successfully utilized in a rodent rhinitis model to help convert a TH2 immune response to a TH1 response. Unfortunately, the immunomodulatory attributes of MSCs have also implicated them in promoting tumor states [44]. The coadministration of melanoma cells with MSCs in a murine model has been shown to give rise to tumors [45]. Paracrine signals secreted by MSCs lead to homing of macrophages and monocytes to tumor beds, which in turn promotes propagation of these tumors. Since ADSCs share multiple characteristics with other MSCs, there exist concerns about their role in tumor state propagation. Moreover, since spheroid architecture has been shown to amplify the cytokines secreted by ADSCs, there is an increased need to scrutinize their effect on tumorigenesis. To date, there has been no reported case of tumor development following ADSC administration (one report was later withdrawn) [46]. While many experiments involving ADSC spheroids are too short to reasonably evaluate neoplastic effects, recent experiments using immunocompromised mice have shown that ADSC spheroids do not lead to any tumor formation even after 1 year following transplantation [47]. These experiments significantly bolster the safety profile of ADSC spheroids.
12.11 Conclusions
Adipose-derived stem cells are an abundant source of stem cells that are relatively easy to obtain. They have been well characterized and demonstrate many properties common to other mesenchymal stem cells. Scaffold-free, three-dimensional, multicellular aggregates of ADSCs capitalize on the interaction between stem cells and the self-generated ECM to augment their paracrine effects. The three-dimensional architecture not only makes ADSCs more responsive to external cues but also improves cell viability in hostile environments. Spheroid structure therefore enhances the attributes of ADSCs and may be an ideal formulation in which ADSCs should be used in clinical scenarios.
References
1.
2.
Katz AJ, Llull R, Hedrick MH, Futrell JW. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26(4):587–603.PubMed








