FOR TOPICAL APPLICATIONS
Authors
Anurag Pande, Ph.D.
Sabinsa Corporation
VP, Scientific Affairs
Dr Muhammed Majeed
CEO Sabinsa Corporation
ABSTRACT
Today both consumers and manufacturers are looking for “greener alternatives” for use in cosmetic and personal care products. Infact, the herbal or natural-based cosmetics are one of the fastest-developing segments in the personal care segment. Major cosmetic brands are shifting towards incorporating natural compounds in their products and consumers can find ever-growing choices in “green” cosmetics.
Use of botanicals in skin or personal care products is not new; they have been used in cosmetics for centuries. In Ayurveda, the Indian traditional medicinal system, which is heavily based on botanicals, there are several examples of plant-derived products for alleviating skin problems or enhancing the skin beauty.
In many instances, modern science has provided the scientific evidence for the activity of botanicals. Structural elucidation using the latest technology has helped to identify the active phytochemicals present in such botanicals and understand their mechanism of action. Botanical extracts are no longer the crude extracts used in the traditional system, but have evolved into highly pure and standardized extracts, their efficacy established with modern cell- and enzyme-based assays.
In the present chapter we look into various key elements that are involved in developing botanicals as cosmeceutical actives. We also describe different extraction techniques that are commercially available for obtaining the extracts of choice, and the role of standardization in developing these botanicals extracts as cosmeceuticals with consistent quality and efficacy. We also would present examples of multifunctional botanicals with focus on their activities, standardization, and applications in cosmetics, along with any clinical study done on them. Apart from this, we discuss the very important subject of sustainability of natural resources for use in cosmeceuticals.
4.1.5.2 Natural and naturally derived actives
4.1.5.4 Role of standardization
4.1.5.5 Multifunctional Botanicals
b. Ellagic acid (from pomegranate)
c. Tetrahydrocurcuminoids (Turmeric extract)
d. Sabiwhite® – 955 tetrahydrocurcumin extract
e. Cococin™ (Freeze-dried coconut water)
f. ForsLean (Coleus forskohlii rhizomes)
g. Cosmoperine® (Piper nigrum fruits)
h. ARTONOX® (Artocarpus lakoocha wood)
j. Ursolic acid (Salvia officinalis leaves)
k. Boswellin® CG (Boswellia extract)
4.1.5.6 Sustainability of Botanicals
Today we live in the age of green consumerism, and the personal care market is no different. Over the last one decade, the natural cosmetic market has shown tremendous growth and has been the fastest-developing segment in the personal care industry. In this consumer-driven market, the shift towards natural cosmetics can also be seen from the fact that many major cosmetics companies are now opening up for including natural products in their product line. The use of natural products in beauty and makeup is not new; examples of it can be seen in several civilizations. Use of oils such as thyme and rosemary on skin by Egyptians is well depicted hieroglyphically. Japanese used safflowers as pigments for rouge and lip makeup for ages (www.jcia.org), and in Indian culture examples of natural cosmetics such as dyeing hair with henna are well known (Auboyer 2002). The following sections are dedicated to discussion on the natural actives or natural cosmeceutical actives.
4.1.5.2 NATURAL AND NATURALLY DERIVED ACTIVES
Though definitions may vary for natural and naturally derived actives, it is important to understand the difference between naturally occurring and naturally derived cosmeceutical actives. Naturally occurring compounds when provided in their natural form can be called natural products; hence epigallocatechin gallate when provided in an extract can be considered a natural active.
On the other hand, naturally derived actives do not occur in nature, but can be synthesized from naturally occurring sources. Examples of such compounds will be discussed in a later part of this chapter.
Since the natural compounds exist in a natural matrix of plant tissues and cells along with thousands of other coexisting compounds, to use them in conventional topical formulations it is important to extract them selectively from their natural matrix and purify them. These natural compounds may be present in certain parts of plant or herbal material, such as leaves, rhizomes, fruits, bark, or heartwood. For extraction of raw material, the process should be carefully chosen. The main requirements of the extraction process are:
- a. Should be compatible with the plant material
- b. Should be capable of selectively extracting and purifying the compound
- c. Should be environmentally friendly
- d. Should not be degenerative to compound or plant material
- e. Should be economically feasible
Based on the raw material, there are various extraction processes that are commonly used in industry. Some of the major extraction processes are discussed below.
- (1) Solvent extraction process
- (2) Freeze-drying process
- (3) Supercritical fluid extraction process
Solvent extraction is the most commonly used process. This process uses a variety of solvents, from a polar to nonpolar range, based on the particular compound of interest. Ethanol, methanol, isopropyl alcohol, ethyl acetate, acetone, and hexane are commonly used for extraction in industrial quantities. These solvents extract selective compounds based on the polarity of the compounds, thus enriching them in the extract.
The freeze-drying process is used for more sensitive and heat-labile compounds, which cannot withstand high-temperature treatment for long period of times, as in the case of solvent-extraction process. Freeze-drying is carried out in three steps: (1) freezing, (2) primary drying, and (3) secondary drying. The temperature of the freezing step is maintained at –40°C, converting most of the water present in the raw material into ice. Primary and secondary drying is carried out at elevated temperature to remove most of the unfrozen water (SM Patel 2010).
Supercritical fluid extraction is a process where supercritical fluids such as carbon dioxide is used for extracting the material from a solid or liquid matrix. Carbon dioxide is the most commonly used supercritical fluid in this type of extraction. Carbon dioxide at its critical temperature (31°C) and critical pressure (74 Bars) tends to behave both like a gas and a liquid. This type of extraction process requires an expensive setup and has limitations in the of types of raw material that can be extracted. Carbon dioxide, being a nonpolar itself, can extract compounds of similar polarity and is thus limited to nonpolar compounds present in oils and oleoresins. Use of modifiers can to some extent increase the range of compounds that can be extracted (He et al. 2005).
4.1.5.4 ROLE OF STANDARDIZATION
One thing that can decide the efficacy of a product is the amount of active inside; thus it is of prime importance that the product should be properly formulated to contain the required amount of active inside. In natural products where the extracts may contain several hundred compounds present in macro- to micro- and even in nano-quantities, it is of utmost importance to have a proper standardization of the extract. Standardization is defined by the American Herbal Product Association (AHPA) as “the body of information and controls necessary to produce materials of reasonable consistency. This is achieved through minimizing the inherent variation of natural product composition through the quality assurance practices applied to agricultural and manufacturing processes” (Gaedcke et al. 2003). The first step in standardization of an extract is identification of the active compound. Once the active component is identified, it can be used as a reference for the quality of an extract and also its dosage levels for formulation. For a good batch-to-batch efficacy, the product should contain the same amount of actives. One of the major tasks in natural product formulation is preparation of a reference library of compounds that are used for standardizing an extract.
Apart from consistency in quality, standardization also helps to preserve the authenticity of the natural extracts. Proper identification of the marker compounds and chemical fingerprinting of the extracts using the latest chromatographic tools help ensure that extracts provide the desired biological activity and are free from any adulterants.
4.1.5.5 MULTIFUNCTIONAL BOTANICALS
Saberry® is the trade name for the Indian gooseberry extract marketed by Sabinsa. Saberry® is standardized to 10% of the active compound Beta glucogallin. Indian gooseberry or Amla is regarded as a tonic herb or rasayan in Ayurveda. It is also known as a detoxifying herb and is used to correct the doshas in Ayurveda. Apart from its use in oral Ayurvedic formulations, Amla is also used in topical beauty care. Amla oil is known to be one of the world’s oldest natural hair conditioners. Amla fruits have also been studied for their skin-lightening activity and MMP-1 inhibitory activity.
Amla fruit were long considered a rich source of vitamin C, which also accounts for its high antioxidant activity. However, the studies carried out on Amla in the last few years have raised questions on the presence of ascorbic acid in the fruits.
In order to unfold the nutritional and cosmeceutical benefits of gooseberry, a proper identification of a valid biomarker was required to resolve the confusion and authenticate the extract. Extensive work done by the research group at Sabinsa using modern isolation and hyphenated techniques like HPLC, flash chromatography, and spectroscopic method LCMS and NMR led to the identification of biomarker compound Beta glucogallin (Figure 1).
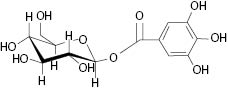
Fig 1: Structure of Beta glucogallin
Amla extract biostandardized with Beta glucogallin revealed the true cosmeceutical potential of this superfruit from India (Majeed et al. 2009).
Cosmeceutical potential of Saberry® (Indian gooseberry extract)
Antioxidant Activity
Brunswick Labs in Wareham, MA evaluated the ORAC activity of Saberry® using state-of-the-art ORAC tests. The ORAC* assays—ORAC, HORAC, NORAC, SORAC, and SOAC—are validated tests to measure broad-spectrum antioxidant power. The ORAC activity is expressed in micromole standard per gram. ORAC analysis (Table 1) showed that Saberry® has excellent ORAC activity in hydrophilic media. As expressed in the table below, Saberry® showed combined ORAC activity of 3586 micromole Trolox Eq per gram.
ORAC Activity
ORAC Hydro (µmol TE/g) | ORAC Lipo (µmol TE/g) | ORAC Total (µmol TE/g) | HORAC
(µmol CAE/g) | NORAC
(µmol TE/g) | SORAC
(Kunits SODeq/g) | SOAC
(µmol VItE/g) |
2678 | 4 | 2682 | 345 | 904 | 102 | 1351 |
Table 1: ORAC activity for Saberry®
ORAC* Activity or Oxygen Radical Absorbance Capacity is an assay to measure the antioxidant activity in biological samples. AOAC International recently approved the ORAC method as a first action official method for measuring antioxidants in foods. (http://www.aoac.org/News/AOAC_SPSFAM-01042013-2.htm). The various ORAC activities mentioned above are part of the ORAC activity assay, namely, ORAChydro measures the activity against peroxyl radicals in hydrophilic medium. ORACLipo measures the antioxidant activity against peroxyl radicals in lipophillic medium. HORAC measures the activity against hydroxyl free radicals, while NORAC measures the antioxidant activity against peroxynitrite radicals. SORAC measures activity against the superoxide free radicals, and SOAC measures the antioxidant activity against the singlet oxygen. |
1. Skin-lightening activity
The skin-lightening activity of Saberry® Amla extract standardized with 10% Beta glucogallin was studied by the research group at Sabinsa Corporation using nonanimal test models. Saberry® was tested for its melanogenesis-inhibitory activity using B16 F1 melanoma cells. The melanin production in these melanoma cells is induced by α-MSH and cAMP cells in two different samples. These cells were then treated with varying concentration of Saberry over a period of nine days with test substance (Saberry®) treatment repeated at regular intervals for three days (Figures 2, 3).
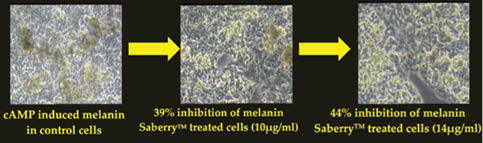
Figure 2: Inhibition of camp-induced melanogenesis by Saberry® in B16 F1 mouse melanoma cells
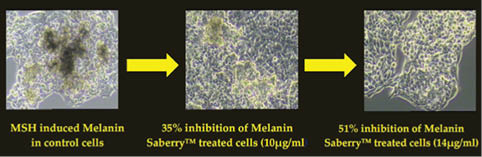
Figure 3: Inhibition of α-MSH–induced melanogenesis by Saberry® in B16 F1 mouse melanoma cells
The melanin thus produced in the cells was treated with 1N NaOH, and absorbance was read at 405 nm in a microplate reader. The study was carried out with comparison to kojic acid.
Saberry showed IC50 of 14µg/ml in both cAMP induced and α-MSH–induced melanogenesis inhibition. Kojic acid in the same test showed IC50 of 100µg/ml (Sami Labs Report 09-223).
2. UVA and UVB protection by Amla extract
Amla extract is known for protecting the human dermal fibroblast cells against oxidative stress. The studies have shown its protective effect on human skin fibroblasts, especially for production of pro-collagen. In a study published in J Ethnopharmacology (2008), it was found that Amla extract stimulated the proliferation of fibroblasts and helped to induce the production of pro-collagen in a dose-dependent manner. Study also showed inhibition of MMP-1 enzymes from the fibroblast cells (Fuji et al. 2008).
MMP-1 or matrix metalloproteinase (MMP) is responsible for the dermal photoaging in human skin. UV irradiation actually up-regulates the production of UV-induced collagenases such as MMP-1 from dermal fibroblast cells (Quan et al. 2010).
Scientists at Sabinsa studied the cell viability of Swiss 3T3 mouse fibroblast cells in the presence of UV exposure with and without Saberry®. The Swiss 3T3 mouse fibroblast cells were placed in a microplate and treated with varying concentrations of Saberry and exposed to 5J cm-2 UV dosage and 0.05J cm-2 UVB dosage. After the exposure, the cells were incubated in a CO2 incubator for 48 hours and developed by NRU staining techniques to analyze the cell viability (Figure 4).
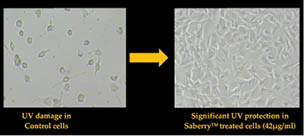
Figure 4: UV protection by Saberry® in Swiss 3T3 mouse fibroblast cells
Saberry® showed IC50 of 14µg/ml for protection against UVA radiation and 42µg/ml against UVB radiation (Sami Labs Report 09-223).
Sami Labs Report: 09 223 Cosmeceutical potential of Saberry®
Cosmeceutical applications of Saberry®
Saberry® is standardized with a unique biomarker—Beta glucogallin. It is a beige-colored extract and completely water soluble. It can be used in anti-aging formulation, skin lightening or skin fairness, and sun-protection formula. It is free from any preservative.
Formulation guidelines
- 1. Solubilize Saberry® before adding it to emulsions below 40°C.
- 2. It forms a hazy solution with water (1:10) at 40°C.
- 3. Maintain the pH of the formulation as slightly acidic (5.0–6.0).
- 4. Saberry® can be gelled using thickeners such as carbomer, acrylates copolymer, and lecithin.
- 5. Protect the finished product from direct exposure to light and heat and use opaque packing.
Suggested level of use
Skin-lightening formulations: 0.1–0.3% w/w
Anti-aging: 0.05–0.2% w/w
Suncare/ after suncare: 0.2–0.3% w/w
Depigmentation: 0.1–0.2% w/w
b. Ellagic acid (from pomegranate)
Ellagic acid (Figure 5) is a naturally occurring polyphenol present in a variety of plants such as cranberries, raspberries, walnuts, grapes, and pomegranates (Usta et al. 2013).
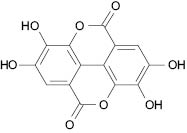
Figure 5: Ellagic acid
Pomegranate as a source of ellagic acid has been of much interest for both nutraceutical as well as cosmeceutical benefits. Pomegranate fruits have shown antioxidant properties due to the presence of ellagic acid, which is one of the main polyphenols in the pomegranate fruit (Bell et al. 2008). Ellagic acid has been shown to have anticarcinogenic, antioxidative, and anti-proliferative activities.
Skin-lightening activity
Ellagic acid has also been studied for its topical benefits. Ellagic acid, being a polyphenol, is able to interfere with oxidative steps in the melanin synthesis, which is catalyzed by the tyrosinase enzyme.
Furthermore, due to its antioxidant activity it can also scavenge the reactive oxygen species, which can also play important role in melanin synthesis (John et al. 2005). Another mechanism proposed for its skin-lightening activity is related to its higher affinity to copper present on the active site of the tyrosinase enzyme (Shimogaki et al. 2000), which catalyzes the oxidative conversion of tyrosine to dihydroxy-phenylalanine (DOPA) and further from DOPA to DOPA-quinone.
To understand the underlying mechanism of tyrosinase inhibition by ellagic acid, Shimogaki et al. studied tyrosinase inhibition by ellagic acid in the presence of copper. While the tyrosinase enzyme activity was reduced or inhibited in presence of ellagic acid, this was partially recovered following the addition of copper. This suggested that the ellagic acid inhibits the tyrosinase activity by affecting the copper present at the active site of the enzyme (Shimogaki et al. 2000).
By incubating the cells with ellagic acid in the presence of copper compounds, researchers were able to show that ellagic acid inhibits the tyrosinase activity by affecting the copper present at the active site of the enzyme.
UV-protective activity
In the animal study done by same group (Shimogaki et al.), the protective activity of ellagic acid against skin pigmentation induced by UV rays was studied and compared to kojic acid and arbutin.
The study was carried out on brownish guinea pigs, which were exposed to UV-B irradiation once a day for eight days on a shorn test site on their backs. The test substance (ellagic acid) was applied topically every day for six weeks. After the six weeks the animals were sacrificed and skin samples were studied. It was found that ellagic acid plays a key role in preventing skin pigmentation following UV irradiation. The melanin content of skin was found to be reduced with ellagic acid application, not only in the basal layer, but also in other layers such as the stratum spinosum, stratum granulaosum, and stratum corneum.
Comparative data showed that ellagic acid at 1% was more efficient than kojic acid and arbutin at same percentage in reducing pigmentation on the skin.
Cosmeceutical application of ellagic acid
Ellagic acid containing extracts can be used for skin lightening, for management of hyperpigmentation, UVB protection, and antioxidant activity. As ellagic acid is not soluble in water, solvents such as PEG 300 and BG can be used to dissolve the active in the formulation stages. Suggested level of use is 0.1–0.5%.
Quasi-drug status of ellagic acid in Japan
Ellagic acid is one of the approved quasi-drug ingredients for management of hyperpigmentation disorder in Japan. Skin-lightening quasi-drug is a category of cosmetics that can be regarded as functional cosmetics, and can prevent or improve hyperpigmentation disorders such as melasma and chloasma. Quasi-drugs require a premarket approval by Japanese ministry of Health, Labor and Welfare (MHLW) (Ando et al. 2010).
c. Tetrahydrocurcuminoids (Turmeric extract)
Tetrahydrocurcuminoids are the major metabolites of the naturally occurring curcuminoids in the turmeric rhizomes. The curcuminoids are the active compounds present in turmeric. curcuminoids have been focus of several research studies and clinical trials for their anti-inflammatory, anti-cancer, hepatoprotective, antioxidative activities etc. (Osawa 2007).
Curcuminoids have also been traditionally used on skin for skin fairness and for healing of wounds. Paste of turmeric and neem is used for ulcers and scabies (Phillip 2011, 2nd ed.). However, due to their dyeing nature, the use of curcumin or turmeric based topical products on skin is quite cumbersome. Application of curcumin on skin can lead to staining that may last for a couple of days, not ideal for conventional cosmetic application for Caucasian skin.
Scientists at Sabinsa found a novel way to introduce the goodness of curcumin/turmeric in conventional cosmetics without including the staining nature of the curcumin.
Tetrahydrocurcuminoids (Figure 6), as mentioned above, are the major metabolites of curcuminoids. Tetrahydrocurcumin is obtained by hydrogenation of the double bonds existing in the curcumin structure. Due to loss of conjugation in the structure (Figure 7), the tetrahydrocurcumin absorbs the light in UV area and appears white in color. Tetrahydrocurcumins are not only free from color, but also from staining nature of curcumin.
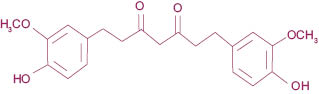
Figure 6: Structure of tetrahydrocurcumin
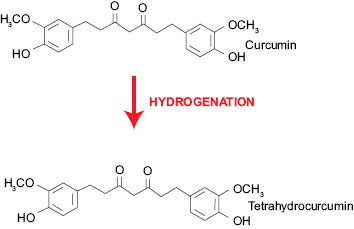
Figure 7: Conversion of curcumin to tetrahydrocurcumin
Tetrahydrocurcumin as an antioxidant
Tetrahydrocurcuminoids are obtained from curcuminoids, which are well known for their antioxidant activity, and hence the studies have been carried out to compare antioxidant activity of both curcumin and its metabolites—tetrahydrocurcuminoids. Studies carried out by Osawa et al. found that tetrahydrocurcumninoids had the strongest antioxidant activity among all the curcuminoids tested in different assay methods.
Osawa et al. selected curcumin, demethoxycurcumin, and bis-demethoxy curcumin and their corresponding hydrogenated tetrahydrocurcuminoids for comparison by using linoleic acid as substrate in an ethanol/water system. They also used in vitro systems such as rabbit erythrocyte membrane ghost and rat liver microsomes for determining the antioxidant activity of these compounds by measuring the TBARS (thiobarbituric acid reactive species) generated by the oxidants (Osawa et al. 1995).
DPPH Free Radical Scavenging activity
Free radical scavenging activity of tetrahydrocurcuminoids was determined using DPPH assay (diphenyl picril hydrazyl radicals). DPPH radicals show a decrease in absorbance due to their quenching by antioxidants.
Different concentrations of test substance are incubated with 0.1mM DPPH methanolic solution for 30 minutes. The reduction in absorbance of DPPH radicals is then studied at 516nm and scavenging activity is expressed as SC50.
Using the above method to determine the free radical scavenging activity, tetrahydrocurcuminoids was compared with resveratrol (95%). The results are given in the table below.
Tetrahydrocurcuminoids were found to have more than twice antioxidant activity than the resveratrol, the major polyphenols present in grapes skin and red wine (Sami Lab Test Report 201).
Product | Scavenging Activity SC50 |
Tetrahydrocurcuminoids 95% | 1.14 |
Resveratrol 95% | 3.24 |
Table 2: DPPH free radical scavenging activity
Skin-lightening potential of tetrahydrocurcuminoids
In view of traditional use of turmeric on skin for increasing glow and radiance, tetrahydrocurcuminoids were evaluated for their skin-lightening potential using an enzyme-based method involving tyrosinase inhibition as well as an in vitro method using melanogenesis inhibition in B16 F1 mouse melanoma cells.
In the enzyme-based assay, tetrahydrocurcuminoid compared favorably to several other skin-lightening natural compounds such as kojic acid, vitamin C, and Arbutin (Sami Labs Report No. 09, 222). The table below gives the tyrosinase inhibition activity in terms of IC50 (Table 3).
Product | Inhibition Capacity IC50 |
Tetrahydrocurcuminoids 95% | 2.0 |
Kojic acid | 3.0 |
Vitamin C | 9.33 |
Arbutin | 33.0 |
Table 3: Tyrosinase inhibition activity
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








