(1)
Department of Orthopaedic Surgery, University of Michigan Hospital and Health Systems, Ann Arbor, MI, USA
Abstract
The use of intravital microscopy in experimental transplantation research has been popular for decades. Siemionow et al. have reported several in vivo studies on the microcirculation of allograft rejection and ischemia/ reperfusion injury using a rat cremaster muscle model. In these studies, the cremaster muscle was used not only as a free flap, but also incorporated into hind-limb allografts as part of the composite tissue. As a result, a pattern of leukocyte trafficking, and sequence of hemodynamic changes were established during the acute rejection of composite tissue allografts. Rat model however, presents certain obstacles. Genetically engineered transgenic and knock-out rat strains are not readily available. Also, number of monoclonal antibodies and reagents are limited in rats. To overcome those limitations, we developed mouse cremaster muscle allograft model. In this chapter, you will find relevant technical details, advantages, and challenges of the model.
Keywords
AllotransplantationCremaster muscleExperimental modelIntravital microscopyMouseSurgical Technique
Human hand can anastomose 250–300 μm vessels [1]. Cremaster muscle pedicle in mice is only 75 μm in diameter, which makes it impossible to anastomose using conventional techniques. In order to harvest the cremaster muscle on vessels that are larger in diameter, we conducted a pilot study measuring vessel diameters proximal to the pudic-epigastric artery (Fig. 16.1). In order to match the size of the recipient vessels, we also measured vessel diameters at inguinal and neck areas for femoral and carotid vessels, respectively. The comparison between the donor and recipient vessels are shown on Table 16.1. Based on those results, we connected iliac vessels with the carotid artery and external jugular vein in the neck region.
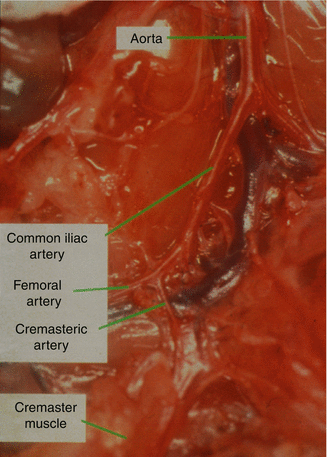

Fig. 16.1
Cremasteric, femoral and common iliac artery of the mouse as a potential pedicle of the cremaster muscle flap
Table 16.1
The closest match is found between the carotid artery/external jugular vein (as recipient) and common iliac vessels (as donors)
Recipient vessels | Donor vessels |
|---|---|
Carotid artery: 250 μm | Cremasteric artery: 75 μm |
Femoral artery: 100 μm | Common iliac art: 250 μm |
Aorta (above bifurcation): 300 μm | |
Aorta (infrarenal): 400 μm | |
External jugular vein: 300 μm | Cremasteric vein: 90 μm |
Femoral vein: 125 μm | Common iliac vein: 300 μm |
Inf. vena cava (bifurc): 350 μm | |
Inf. vena cava (infrarenal): 500 μm |
Preparation of the Donor
Following induction of anesthesia and skin preparation, we made an anterior longitudinal skin incision starting from testicles to the xyphoid process of the sternum. The cremasteric muscle was identified on its vascular pedicle. Through an anterior incision at the superior pole of the muscle, the testicular vessels were ligated and the testicular contents were extracted. The cremaster muscle was isolated as a tube flap (Fig. 16.2a). Following ligation of all side branches coming off of femoral and iliac vessels, the tube flap was elevated on the iliac artery and the vein (Fig. 16.2b).
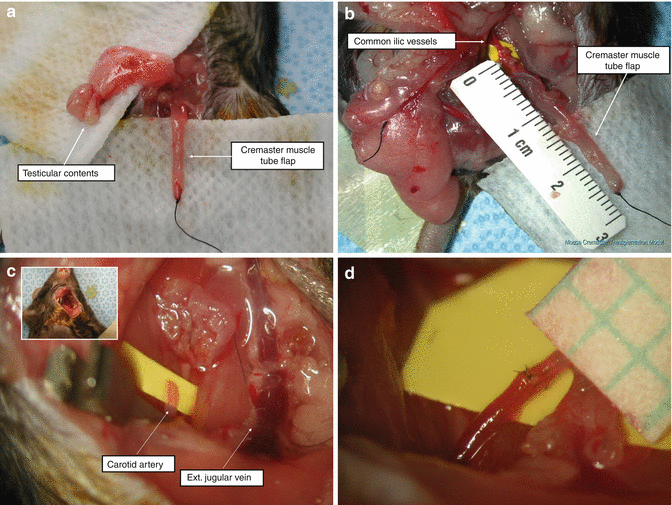

Fig. 16.2
(a) A cremaster muscle allograft is shown following extraction of the testicular contents. (b) Approximately 15 mm long flap pedicle. All of the side branches are ligated. (c) The sternocleidomastoid muscle is detached from the clavicle to provide broader exposure to the carotid artery and external jugular vein. (d) Completed vascular anastomosis between the iliac and carotid arteries
Preparation of the Recipient
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








