The aging midface has long been overlooked in cosmetic surgery. Our understanding of facial aging in terms of 3 dimensions has placed increased importance on volume restoration. Although an “off-label” indication for most fillers in this facial region, volumization of the midface with injectable fillers is usually a safe and straightforward procedure technically. Injectors, nevertheless, need to have an excellent understanding of facial anatomy and the characteristics of the injected products should problems arise.
Key points
- •
Aging changes of the midface include loss of bony support, facial lipoatrophy, and descent of soft tissues.
- •
Restoration of midface volume can be achieved by the use of injectable filling agents.
- •
The improvement in lower lid and midface volume achieved with the use of filling agents can produce results lasting up to 2 years.
- •
Injection techniques in this region may vary and can include the use of needles or cannulas for product placement.
- •
Complications of soft tissue filler injections to the midface are generally mild and self-limiting.
Introduction
In recent years, our understanding of the changes associated with facial aging has improved. Volume deficit, rather than vertical descent or ptosis alone, is now appreciated as a critical component of facial aging. We understand volume loss in terms of bony remodeling of the craniofacial skeleton, lipoatrophy of the fat pads, and loss of skin and muscle tone. In addition, the skin of the aging face loses volume and elasticity. In the midface and periorbital area, loss of volume results in a number of changes characteristic of the facial aging process. The tear trough and lid–cheek junction become more visible, and the malar region flattens and descends.
Our focus on facial rejuvenation has added the 3-dimensional idea of volume restoration to the traditional 2-dimensional process of surgically repositioning ptotic tissues. Volumizing the midface may be accomplished with either autologous fat or currently available filling materials. In the early 2000s, new classes of filling agents were developed that proved to be superior to collagen in both effect and duration of results. Many such filling materials have since gained tremendous popularity in the nonsurgical treatment of volume loss seen with facial aging. A number of filler materials, including hyaluronic acid (HA), calcium hydroxylapatite (CaHA), polymethyl methacrylate (PMMA) and poly- l -lactic acid (PLLA), are now available in the United States.
Introduction
In recent years, our understanding of the changes associated with facial aging has improved. Volume deficit, rather than vertical descent or ptosis alone, is now appreciated as a critical component of facial aging. We understand volume loss in terms of bony remodeling of the craniofacial skeleton, lipoatrophy of the fat pads, and loss of skin and muscle tone. In addition, the skin of the aging face loses volume and elasticity. In the midface and periorbital area, loss of volume results in a number of changes characteristic of the facial aging process. The tear trough and lid–cheek junction become more visible, and the malar region flattens and descends.
Our focus on facial rejuvenation has added the 3-dimensional idea of volume restoration to the traditional 2-dimensional process of surgically repositioning ptotic tissues. Volumizing the midface may be accomplished with either autologous fat or currently available filling materials. In the early 2000s, new classes of filling agents were developed that proved to be superior to collagen in both effect and duration of results. Many such filling materials have since gained tremendous popularity in the nonsurgical treatment of volume loss seen with facial aging. A number of filler materials, including hyaluronic acid (HA), calcium hydroxylapatite (CaHA), polymethyl methacrylate (PMMA) and poly- l -lactic acid (PLLA), are now available in the United States.
Treatment goals
Given our current understanding of the central role of volume loss in facial aging, facial rejuvenation techniques have shifted away from treating wrinkles alone and toward increasing volume while shaping and sculpting the face. Injectable fillers can be used to restore volume and reduce the appearance of wrinkles and folds, thereby creating a more youthful appearance. Injectable fillers are especially useful in midface and periorbital rejuvenation, because many of the effects of aging in these areas are owing to volume depletion.
The specific goals of treatment must be ascertained for each individual patient. The selection of filler material and placement, as well as injection technique, must then be tailored for each patient, taking into account the specific anatomic changes observed in that individual, age and medical comorbidities, willingness for downtime, and cost.
Pretreatment assessment
The boundaries of the midface are the inferior orbital rim superiorly, the nasal sidewall medially, and the nasolabial fold inferiorly and along the course of the zygomaticus major muscle laterally ( Fig. 1 ). Understanding of facial anatomy and of the contributions of different anatomic components to overall facial aging in each specific patient is critical in the selection and use of injection fillers. Careful assessment of each tissue layer of this region is paramount in determining treatments for facial rejuvenation, to obtain natural-looking results.
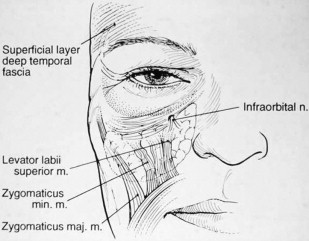
The lower lids and midface should be analyzed with the patient in a seated and upright position. Attention should be directed toward the degree of skeletonization of the lower lids, degree of orbital fat herniation, midface volume, and soft tissue ptosis. Fillers can be placed in the lower lid and/or the cheek to rejuvenate the midface, similar to the placement of autologous fat.
Lower Lid
The lower lid complex consists of skin, fat, and bone. In the lower lid, the tear trough refers to the depression extending from the medial canthus. It separates the lower eyelid from the cheek and is bound superiorly by the orbital fat pad. The tear trough ends approximately at the midpupillary line. Extending laterally from this point is an additional groove called the lid–cheek junction, which is roughly parallel to the infraorbital rim. The muscle components of both the tear trough and lid–cheek junction are fixed to bone; inferior descent of these structures is therefore minimal. The tear trough and lid–cheek junction instead become more visible with age because of volume loss. In addition, with aging, the central portion of the orbicularis-retaining ligament becomes more lax, resulting in inferior displacement of the orbital fat pads. Herniation or pseudoherniation of the fat pad above produces lower lid “bags,” deepens the tear trough, and further accentuates the trough by creating shadowing beneath. The inferior orbital rim becomes skeletonized and these changes produce the tear trough deformity.
The tear trough and lid–cheek junction are also defined by differences between the eyelid and cheek skin. The skin of the lower eyelid is thinner and more pigmented than that of the upper cheek; the upper cheek also has a thicker dermis and more underlying subcutaneous fat. With time, lid skin can become even more pigmented, increasing the contrast between the lid and the upper cheek.
Evaluation of each of these components is critical before proceeding with lower lid volumization with injectable fillers. Ideal candidates have thick skin and are without large orbital fat pads. Patients with significant herniation of orbital fat may instead benefit more from surgery, because fillers alone may not eliminate adequately the transition between the protuberant fat pad and the concave tear trough.
Midface
Evaluation of the midface for volumization should include assessment of both the soft tissue and bony components of the region. Fat in this area exists in several well-defined compartments in superficial and deep layers. With facial aging, there is volume depletion in these compartments. There is also ptosis and migration of the fat compartments. As volume is lost and tissues descend, the malar bags become prominent owing to the tethering of the zygomaticocutaneous ligament. The skin also becomes ptotic. In addition, bone remodeling of the midface and orbit contributes to the change in appearance of the overlying soft tissues. As a result of these changes, the full “apple cheeks” of youth become deflated. Ideal candidates for midface volume restoration have a minimal to moderate tear trough deformity and diminished volume in the cheeks, with minimal to moderate soft tissue descent.
Product selection
Lower Lid
Volume restoration of the tear trough with filler materials is a difficult technique to master and should only be performed by well-trained practitioners. The lower lid region may be particularly difficult to inject, given its thin skin and its proximity to the orbital and malar fat pads. HA products are widely used in this area given their safety profiles, low allergic potential, and relative ease of injection. Furthermore, HA fillers can be dissolved with hyaluronidase, another advantage when treating this unforgiving region of the face.
In one author’s opinion (T.K.), only 2 Food and Drug Administration (FDA)-approved HA fillers—Restylane (Galderma Laboratories, Fort Worth, TX) and Belotero (Merz Aesthetics, Greensboro, NC)—are appropriate fillers to use in this region. The thin, syruplike nature of Juvederm combined with the motion of the orbicularis muscle results in pooling of the product over time. Restylane, however, has a more ideal consistency for placement in the lower lids ( Fig. 2 ), although superficial placement or migration of the product may result in a bluish hue under the skin ( Fig. 3 ). This discoloration is referred to as the Tyndall effect, which is believed to result from the refraction of blue light by the uniformly sized Restylane particles. Occasionally, hyaluronidase injections are necessary to correct this deformity by rapid enzymatic degradation of the product.
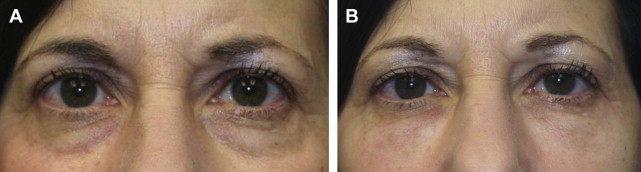
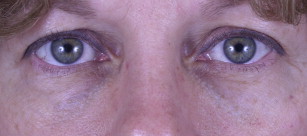
Belotero, on the other hand, is manufactured by a different process (CPM technology), which creates a polydensified matrix. This produces a product with more uniform particle size than Restylane, which allows it to spread more easily. It may therefore be injected more superficially and is less likely to result in the Tyndall effect ( Fig. 4 ). Unlike Restylane, Belotero does not have lidocaine added to the product. Because of its hydrophilic nature, undiluted Belotero can result in significant edema of the lower lids; dilution of the product by the addition of lidocaine just before injection is therefore suggested.
Midface
Volumization of the malar region is technically simple, and a variety of fillers are appropriate for this region. HA, CaHA, PLLA, and PMMA have all been used to augment the midface with good results. However, only 1 filler, the HA product Juvederm Voluma (Allergan, Irvine, CA), has been FDA approved to treat this region.
Hyaluronic acid
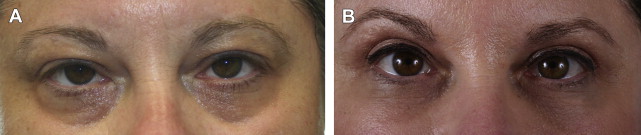
HA is a naturally occurring polysaccharide found in the skin, cartilage, synovial joint fluid, and other connective tissues and is identical in structure across all mammalian species. It is an ideal filler because it is biodegradable and nonimmunogenic. There are many HA filler products available; they differ in their degree of cross-linking, gel consistency, and concentration. They have been shown to have good safety profiles and excellent outcomes ( Fig. 5 ).
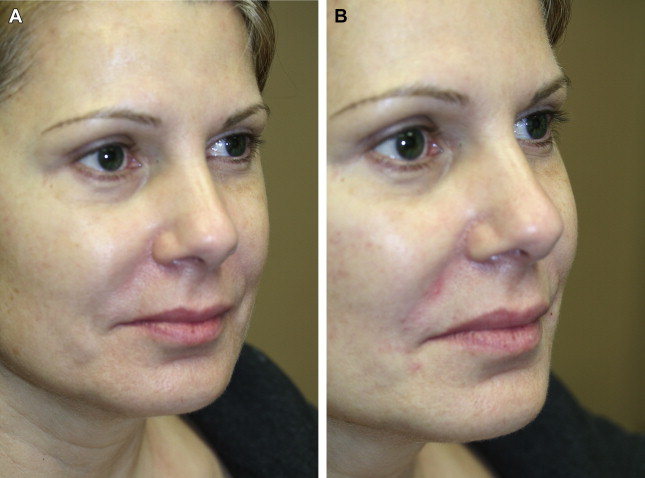
Injection of HA fillers may be performed using 27- or 30-gauge needles. Alternatively, cannulas may also be used. This author (T.K.) prefers to use an injection of 1% xylocaine with 1:100,000 epinephrine at the needle entry site. The cannula is then inserted through a control hole made with a 25-gauge needle. The product is then feathered in the deep subcutaneous tissue or preperiosteal plane.
Juvederm Voluma is a newer filling material that received FDA approval in late 2013 for midface volumization. The pivotal study by Jones and Murphy showed it was safe and effective for up to 2 years. The injection technique for Voluma begins with injections in the upper outer quadrant, proceeding inferomedially to give volume to the cheek.
Calcium hydroxylapatite
Radiesse (Merz Aesthetics) is composed of spheres of CaHA in an aqueous gel carrier. Unlike HA, Radiesse works as a volumizer by stimulating production of collagen; the microspheres act as a scaffold for the newly formed collagen. The carrier gel is resorbed within several weeks after injection, and the CaHA itself slowly degrades over time and is excreted by the body. Like HA, CaHA is nonimmunogenic and biodegradable, with a favorable safety profile. Radiesse works well to fill the midface because it has a high lift capacity and results may persist for about a year ( Fig. 6 ).





