(1)
Department of Plastic and Aesthetic Surgery, Solumed, Poznan, Poland
(2)
Department of Orthopaedics, University of Illinois at Chicago, Chicago, IL, USA
Abstract
Cigarette smoking delays wound healing and is associated with higher complication rates after reconstructive and aesthetic procedures including microsurgery and free tissue transfer, arterial reconstruction, subcutaneous mastectomy, breast reconstruction, and facelift surgery. One mechanism shown to prolong flap survival in many clinical situations and animal models is sympathectomy, which is one of the proposed components of the delay phenomenon. The purpose of study presented in this chapter is assessment whether sympathectomy has the potential to improve the survival of muscle flaps during acute cigarette smoking. We used the rat cremaster muscle flap model, which allowed us to examine the acute effect of cigarette smoking on the microcirculation of both innervated and denervated axial muscle flaps, including changes in hemodynamics and leukocyte behavior. The important finding in this study was the significant difference in vessel behavior during and after smoking when comparing the innervated and denervated groups.
Keywords
MicrocirculationsSmokingFlapsSympathectomyDelay phenomenonCremaster flapIntroduction
Cigarette smoking delays wound healing and is associated with higher complication rates after reconstructive and aesthetic procedures including microsurgery and free tissue transfer, arterial reconstruction, subcutaneous mastectomy, breast reconstruction, and facelift surgery [1, 2]. Smoking causes these negative effects by increasing platelet aggregation, decreasing oxygen transport due to carbon monoxide hemoglobin, and reducing microcirculatory flow through a combination of macroangiopathy, microangiopathy, and vasoconstriction [3–5].
Carbon monoxide binds to hemoglobin with a 200-fold higher affinity than oxygen, which decreases oxygen delivery to tissues primarily through two mechanisms; (1) a decrease in the total available hemoglobin-binding sites for oxygen, manifested as a decrease in PaO2, and (2) an increase in the affinity for oxygen of the remaining binding sites, revealed as a shift in the oxygen dissociation curve to the left. Several other mechanisms also contribute. Hypoxia increases fibrinogen levels and red blood cell aggregation [6]. Nicotine causes both an increase in catecholamine production by the adrenal glands and a local increase in catecholamines in the skin flaps of pigs [7]. Catecholamines produce stimulation of the adrenergic alfa1 receptors, leading to vasoconstriction in the vascular smooth muscle of skin and splanchnic tissues [8]. All these effects of cigarette smoke may play critical roles during free tissue transfer operations; it is thus of interest to investigate ways to improve surgical results for cigarette smokers.
One mechanism shown to prolong flap survival in many clinical situations and animal models is sympathectomy, which is one of the proposed components of the delay phenomenon. In this phenomenon, tissue flaps that are prepared for transfer 2–3 weeks before the transfer its self show better survival. In a delayed flap transfer, the flap is created following the usual procedure. Then, the perivascular adventitia is stripped and/or an accompanying nerve is transected to create sympathectomy. Finally, the tissue is returned to its native location for a 2- to 3-week delay before the final transfer.
The purpose of study presented in this chapter is to assess whether sympathectomy has the potential to improve the survival of muscle flaps during acute cigarette smoking. We used the rat cremaster muscle flap model, which allowed us to examine the acute effect of cigarette smoking on the microcirculation of both innervated and denervated axial muscle flaps, including changes in hemodynamics and leukocyte behavior.
Materials and Methods
This study was approved by the Animal Research Committee of The Cleveland Clinic Foundation. Twelve male Sprague-Dawley rats weighing from 150 to 220 g were divided among two experimental groups as innervated and denervated muscle flaps (plus parmeability control group n = 6 which was not exposured to cigarette smoke and in vivo microcirculatory observations). The animals were caged individually, and their environment was maintained at room temperature with a 12-h day/night (light/dark) cycle. Standard laboratory food for tats and water were provided to the animals freely. After microcirculatory observations were completed, the animals were euthanatized with intracardiac injections of pentobarbital.
Flap Preparation
Anesthesia was induced with intramuscular injections of ketamine (5–10 mg/kg) and xylazine (40–80 mg/kg). In both groups, rats underwent dissection of the right cremaster muscle as an axial muscle flap under a Zeiss OPMI 6 operating microscope. A skin incision was made from the anterior iliac spine to the tip of the scrotum for the cremaster preparation. The right testicle and surrounding cremaster muscle and fascia were dissected from the scrotal wall. The cremaster muscle fascia enveloping the muscle and testicle was removed. In the animals assigned to the denervated group, the genitofemoral nerve was identified at the base of the cremaster and a 1-cm segment was resected. In the innervated group, the genitofemoral nerve was left intact. In both groups, thermal cautery was used to create an opening in the muscles anterior, and the testis with the spermatic cord was removed from the cremaster pouch. The spermatic cord and spermatic vessels were ligated, transected, and discarded. The neurovascular pedicle of the cremaster muscle was dissected up to its origin at the external iliac artery and vein.
Preparation for In Vivo Microcirculatory Observations
A rat with a dissected cremaster muscle was placed on a transparent Plexiglas stand in the supine position. The exposed cremaster muscle was then spread out like a sheet upon a glass slide with six to eight 6-0 silk sutures. The muscle was kept moist with Ringer’s solution sealed with oxygen-impermeable plastic film (Saran wrap soaked for 24 h in distilled water). A heating lamp was used to maintain the animal’s temperature and keep the system between 35 and 37 °C. The Plexiglas frame was then placed upon the microscope stand for the intravital observations and measurements of vessel diameter, red cell blood velocity, and leukocyte behavior. This system allowed the cremaster muscle to equilibrate in a controlled environment (Fig. 13.1).
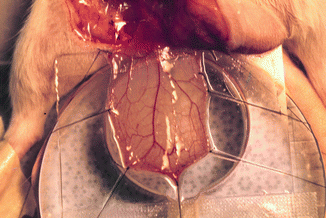

Fig. 13.1
Cremaster muscle flap model under intravital microscopy system. Cremaster muscle after surgical preparation was prepared on the tissue bath for monitoring of microcirculatory hemodynamic changes after exposure to the cigarette smoking
Cigarette Exposure
After the first hour of intravital measurements, the rats were returned to wire mesh cages to be exposed to cigarette smoke. We used a smoking chamber provided by the University of Kentucky Tobacco Research Program (Fig. 13.2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








