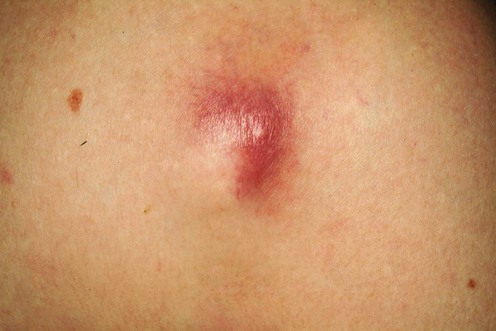
Methicillin-resistant Staphylococcus aureus (MRSA)
Specific investigations
First-line therapy
Dose of trimethoprim–sulfamethoxazole to treat skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



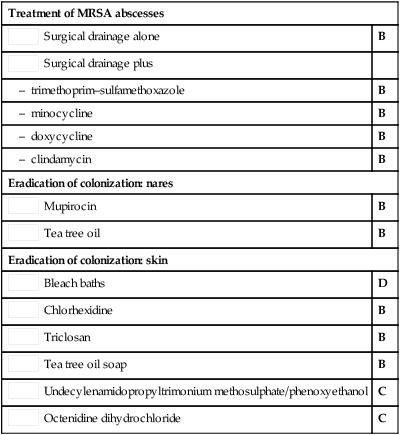
 Surgical drainage alone
Surgical drainage alone Surgical drainage plus
Surgical drainage plus Mupirocin
Mupirocin Tea tree oil
Tea tree oil Bleach baths
Bleach baths Chlorhexidine
Chlorhexidine Triclosan
Triclosan Tea tree oil soap
Tea tree oil soap Undecylenamidopropyltrimonium methosulphate/phenoxyethanol
Undecylenamidopropyltrimonium methosulphate/phenoxyethanol Octenidine dihydrochloride
Octenidine dihydrochloride