Chapter 64 Management of pain and other discomforts in burned patients
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
The words ‘burn injury’ trigger immediate and vivid images of excruciating pain and suffering. Children are conditioned from early childhood that burn injuries are painful and can cause great harm. Until recently, debate continued over the importance of pain management in the burn survivor. Many practitioners believed that the treatment of pain, especially in children, was more dangerous than leaving it untreated. The past 15 years have shown that children’s pain can be effectively and safely managed.1–3 There is mounting evidence that effective pain management leads to a better long-term outcome. Kavanagh et al.4 demonstrated that pain in a burned patient adds significantly to the physiologic demands caused by stress. Successful pain management can significantly reduce the occurrence of psychological disorders such as depression and post-traumatic stress disorder.2,5,6
Pathology of a burn injury as it relates to pain
All burn injuries are painful. Even first-degree burns can produce at least mild pain and discomfort, especially when something such as clothing rubs against the burned area. Second-degree moderate to deep partial-thickness burns result in variable amounts of pain depending on the amount of destruction to the dermis. Superficial dermal burns are the most painful initially. Even the slightest change in air currents moving past the exposed superficial dermis usually causes a patient to experience excruciating pain. Without the protective covering of the epidermis, nerve endings are sensitized and exposed to stimulation. In addition, as the inflammatory response progresses with the increase in swelling and the release of vasoactive substances, pain is increased and begins to involve the surrounding area.7
Pain-generating mechanisms during an initial injury
Studies of humans and monkeys confirm that burn injuries not only make an injured area and surrounding tissue more painful, but also cause hyperalgesia.7–9 Sensory nerve damage may also play a role in excessively difficult to treat pain. Burn injury is characterized by release of large amounts of inflammatory factors such as interleukins, which probably add to the perception of pain and the hyperalgesia. The hyperalgesia is further enhanced because the burn wound heals slowly over days or weeks.
Ptacek et al.10 found that, while there was a general trend for pain to decrease over time, there was also considerable variability in the course of pain among adult burn survivors. Persons with large burns showed a higher affective (suffering) component to the pain, but there is no reliable correlation between the pain scores and burn size. A source of confusion concerning the amount of pain expressed by burn patients is the role played by psychological problems such as anxiety and depression. Choinière et al.11 noted that pain at rest was significantly and positively related to levels of anxiety or depression, i.e. with elevated anxiety or depression, pain scores at rest increased. Although these studies demonstrate the great variability in pain expression in burned patients, they do not identify pain-generating mechanisms, either physiologically or psychologically, in a burn-injured patient. Charlton et al.12 used the State/Trait Anxiety Inventory to measure anxiety and reported that the study sample of adult burned patients was not particularly anxious. Other studies have suggested that burned patients have increased levels of anxiety, especially related to treatment and outcome and that these levels may increase over time.13–16 Anticipation of pain related to wound care that occur at least daily can increase a patient’s perception of pain which in turn can lead to greater anxiety. This reaction may explain some findings which suggest that pain increases over time in burned patients.17–19 Depression also plays a similar role in enhancement of pain.20 Pain leads to depression and depression enhances the perception of pain.5,6 In burn care units, where very aggressive pain management is practiced, depression is not a major problem.21
Pain as a function of the healing process
As a deep dermal or full-thickness burn wound heals, either by primary intention from excision and grafting or by secondary intention through granulation tissue and scar formation, the injured neural tissue is reorganized.18 Reflex neural function returns to grafted burn skin approximately 5–6 weeks after the burn has been covered by autografted skin.19 Active vasodilatation, vasoconstriction, and pain sensation all return at this time. These functions also return to the burn wound which heals through scar formation but may take up to 6 months for complete neural reorganization. This is the basis of neuropathic pain in the burn wound site.
Although rare, causalgia, dysesthesia, and phantom pain syndrome can sometimes develop in healing skin. Phantom limb sensation and pain is more common following amputation, which is often associated with large burn injury or electrical injury. The incidence of these chronic pain syndromes seems to be related to the healing process. Burns that have been excised and grafted on a clean and uniform vascular bed rarely develop one of these chronic pain syndromes. Wounds that heal by granulation and scar formation seem to be more apt to develop a chronic pain problem because of the continued stimulation of nerve fibers in the area with enhancement of the hyperalgesia. Skin biopsies of granulation tissue have clearly shown neuronal tissue entrapment.19 Pain, in scar tissue, subsides over time as the scar tissue matures.
Measurement of pain in burned patients
Pain measurement techniques for an adult burned patient
A variety of pain measurement techniques have been used with adult burned patients. The more common measures include adjective scales (Table 64.1), numeric scales (i.e. rating pain on a scale of 0–5, 0–l0 or 0–l00), and visual analog scales (Fig. 64.1). Each of these scales measures the sensory component of a patient’s pain. Adjective scales and numeric scales are quick and easy to administer because they do not require a visual representation of the scale. The visual analog scale requires a visual representation of the scale to be presented to a patient. Patients must mark or point to the place on the scale that represents their level of pain. The demonstrated validity of the scale allows for comparisons of visual analog pain assessments between studies with different patient samples.
Table 64.1 Adjective scales in English and Spanish
| 0 No pain | 0 Nada de color |
| 1 Slight pain | 1 Dolor leve (ligero) |
| 2 Moderate pain | 2 Dolor moderado |
| 3 Severe pain | 3 Dolor severo |

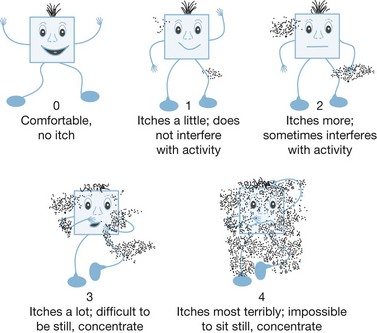
Figure 64.1 Visual analog scale (VAS) for children to rate their levels of pain.
(From the Varno/Thompson Pediatric Pain Questionnaire. With permission from the American Society for Clinical Pharmacology and Therapeutics.)
Motivational-affective and cognitive-evaluative components of pain are most frequently measured using the McGill Pain Questionnaire (MPQ).22 The MPQ takes 10–20 min and consists of 20 sets of adjectives which describe all three components of pain: sensory, affective, and evaluative. Qualitative profiles and quantitative scores for each dimension as well as a total pain score can be derived from the selected adjectives. The MPQ has been translated into several languages and has been shown to be a reliable and valid measurement tool. Gordon et al.23 in a prospective multicenter study, asked 40 adult burned patients to rate their pain on four scales: a visual analog scale; an analog chromatic scale;24 an adjective scale, and a faces scale.25 At the end of the study patients were asked to choose their preferred scale. The majority of subjects preferred the faces and analog chromatic scales. Although further research is needed to validate these findings, the preference of patients is another variable to be considered.
Pain measurement techniques for pediatric burn patients
The measurement of children’s pain is much more complex than it is for adults, especially for pre-verbal children. The assessment of pain in children has included physiologic measurements, behavioral assessment, and patient reports of pain. The physiologic indicators which have been evaluated are heart rate,26 respiratory rate,26 blood pressure,26 endocrine changes,26,27 and changes in PO2.28 None of these shows promise as an indicator for measuring pain in sick children, since all are affected by a variety of stressors, metabolic changes related to a burn, and medications, in addition to pain.
Behavior scales have been devised to measure pain by providing standardized instructions and guidelines for observing behaviors thought to be specific to pain. A number of investigators29–31 have looked at infants’ cries as measurable behaviors that can be observed in order to evaluate pain. These studies demonstrate that length of cry, pitch, intensity, and other characteristics of crying may be used to evaluate pain in infants. Likewise, infant facial expressions may be used to code pain; a system was developed which characterizes nine facial actions involved in the expression of pain, but its use requires videotaping and detailed analyses of an infant’s facial movements.31–33 Both the measurement of crying and facial expression require too much time and special equipment to be appropriate for the clinical setting. Other investigators have devised multidimensional scales that include length of cry, facial expressions, and behavioral states in order to measure pain in infants.34,35 These scales are easier to use and allow an observer to assess pain as either present or absent without further quantification.
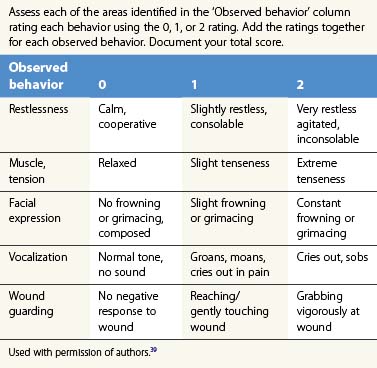
Examples of observational scales, which allow for quantification and may be used with toddlers and pre-verbal children, are the CHEOPS (Children’s Hospital of Eastern Ontario Pain Scale)36 and the Observer Scale.37 The CHEOPS is a scale of six behaviors, each scored on a numeric range; it yields a total numeric score for pain. This scale has been shown to be valid and to have good interrater reliability. The Observer Scale is another standardized instrument that categorizes overall pain or comfort behaviors on a scale of 1–5. The Observational Pain Assessment Scale (OPAS), a burn specific observational tool, was developed by Barone et al. at Shriners Hospital for Children, Cincinnati and is very useful in children 0–3 years of age.38 The scale is depicted in Table 64.2.39
Research suggests that simple self-report scales can be used with preschool children. Examples of such scales include the Oucher Scale (photographs of children with various facial expressions).40–42 Drawings of faces25,43 have also been used with preschool age children44 and school age children (8 years).39 Preschool children have also used the Poker Chip Tool,45 color scales,46,47 and a thermometer47 to report the degree of pain or hurt. These simple tools allow a preschooler to report pain and are easy to use. One caution with the face scales is that a practitioner must help a child differentiate between physical pain and sadness unrelated to pain. Since there is no evidence that any one of these is more valid than another, it is recommended to pick one and use it consistently. When self-report scales are used in conjunction with observational scales, a practitioner gets a better picture of a child’s response to pain and pain therapies.
A school-aged child’s cognitive development allows more abstract thinking. In addition to the Faces Pain Rating Scales which they enjoy,25 they can use simple numeric scales 0–5 in the early school years (ages 7–8) and more complex scales 0–10 or 0–100 in the later years (age 9–12). Visual analog scales anchored with happy and sad faces and simple adjective scales also can be used with this age group.47,48 In addition to self-reports of pain, observational scales such as the CHEOPS36 or the Procedure Behavior Check List49 can be used with a school-aged child. Again, the important issue is to use one selected scale consistently since no one has been shown to be more valid than others.
Intubated and sedated children provide more challenges in the assessment of pain. The more disabilities that a child has and the more medications that are being given to the patient create challenges to the clinician. A 2-year-old child who is blind, with only one extremity that is functioning and who is on numerous medications, presents a huge assessment challenge to the clinician. Box 64.1 presents a list of clinically useful tools according to patient age.
‘Pain is what the child says it is.’53 What about the case where the care-provider documents a lower number than the child says it is because the care-provider believes the child is over-rating the score? Reiman et al.54 surveyed nurses’ knowledge and attitudes regarding pain and their ability to manage pain. The modified Pediatric Nurses’ Knowledge and Attitude Survey regarding pain tool (PNKAS – Shriners Version 2002) needs further validation but demonstrates the need to consider the healthcare provider’s attitude and knowledge of pain.
Measurement of anxiety
Anxiety is measured in a variety of ways. In 2000, Robert et al. surveyed 64 burn treatment centers to determine how they evaluated and treated anxiety, especially in children.50 They found that most centers did not use standardized measures of anxiety.55 Based on that survey and other information, the Shriners Hospital for Children in Galveston has been using the Fear Thermometer adapted by Silverman and Kurtines56 from the Walk’s Fear Thermometer.51 That instrument is illustrated in Figure 64.2.
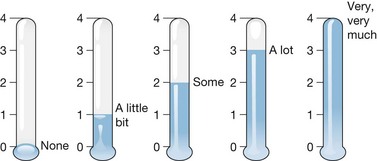
Figure 64.2 Fear thermometer to rate anxiety level.
(From Silverman and Kurtines W. Anxiety and phobic disorders: a pragmatic approach. New York: Plenum; 1996 with kind permission of Springer Science and Business Media.)
Taal et al. introduced a tool to measure burn specific pain anxiety scale (BSPAS).57 This tool is a 5-item scale used to measure anxiety associated with anticipated procedural pain in adult patients. Initial reliability, validity and utility studies have been completed.57,52 A similar tool is needed for children.
Measurement of itching
Itching is very common in burn survivors. Even in small burns, the prevalence is 35% with moderate pruritus and 14% with severe and in many cases the pruritus impacts daily living.58 Another series of 510 burns reported a prevalence of 87%.59 Patients who experience such itching often excoriate new grafts or recently healed skin, thus enhancing their susceptibility to infections. When the pruritus is severe, patients can focus on nothing else. Field et al.60 reported using a visual analog scale of 1 to10 to assess itching. Pat Blakeney, PhD and Janet Marvin, RN at the Shriners Hospital for Children in Galveston teamed up to develop an instrument to measure itchiness, called ‘itch man’ (Fig. 64.3). This instrument was based on a patient’s drawing of his experience in the hospital.61 Children seem to be able to relate to ‘itch man’, and validation has been completed.62 Matheson et al. used a 5-point descriptive itch rating scale in a study comparing effectiveness of shower and bath oil treatments for severe itch, but that scale does not seem suitable for children and is not validated.63
Treatment considerations
• Emergency or resuscitative phase (0–72 h after injury)
• Acute phase (72 h to 3 or 5 weeks, until the wounds are closed)
• Rehabilitative phase (from the time of wound closure to scar maturity). This phase may last months to years.
Pharmacologic management of pain
Pharmacologic management of burn pain is the mainstay of therapy. General rules are helpful in governing the use of pain medication. The first tenet is that if the patient says he/she is having pain, he/she is suffering. The second tenet is that analgesics are most effective when given on a regular scheduled basis (not ‘as needed’ or PRN). Third, pain medication should not be given as an intramuscular injection, since injections themselves cause pain and anxiety, and almost never present an advantage over other routes of administration. Lastly, dose and type of medication should be re-evaluated frequently to make sure pain is continuously controlled and that the patient is experiencing no serious side-effects. The dosing of medication should be adjusted for the general clinical condition of the patients, considering factors of nutritional state, shock, sepsis, age extremes, and concurrent illnesses such as hepatitis. Review articles1,2,64–69 during the last 14 years recommend a variety of therapeutic modalities for background and procedural pain during each phase of burn treatment.
Since the pharmacologic management of burn pain not only spans the phases of burn care, but also must be tailored to meet the needs of all age groups, it is important to comment on pain management in both the very young and the elderly. There is a tendency to give less medication to young children because ‘they don’t complain of pain.’ This opinion is supported by the fact that some children become very withdrawn rather than screaming when they are receiving painful stimuli (e.g. abused children). It is unlikely that a burn is any less painful in a 3-month old than in a 30-year-old; thus, the same therapeutic modalities adjusted for age and size are appropriate for burned children. A major issue of treating pain in young children is the safety risk of using opiates. One example is a 1998 report of the Seattle group concerning respiratory depression in three children treated with standard doses per kilogram of opiates given to other age groups.70 In all cases, the respiratory depression responded well to an opiate antagonist such as naloxone. Young children may have an increased sensitivity to the respiratory depressive effects of opiates.71
Table 64.3 provides a matrix summary of therapies that are recommended for procedural and background pain over the three phases of burn care. A variety of routes or methods of administration are also suggested. The routes or methods recommended include the intravenous bolus (IVB); intravenous continuous infusion (IVCI); patient-controlled analgesia (PCA), and orally administered agents on a time interval rather than pain contingent (non-pain contingent; NPC) basis. Whether more or less morphine ends up being given is not clear. However, many patients especially adolescents, greatly prefer being in charge with a PCA.
Table 64.3 Pharmacologic therapies for burn pain relief
| Emergent phase | ||
| Procedural analgesics | Background analgesics | Anxiolytics |
| Morphine (IVB, IVCI) | Morphine (IVCI, PCA) | Diazepam (IV) (Valium) |
| Meperidine (IVB) | Meperidine (PCA) | Lorazepam (IV) (Ativan) |
| Fentanyl (IVB, IVCI) | Methadone (PO, NPC) | Midazolam (IV, IVCI) (Versed) |
| Hydromorphone (IVB, PO) (Dilaudid) | ||
| Nalbuphine (IVB) (Nubain) | ||
| Ketamine (IV) (Ketalar) | ||
| Nitrous oxide (IH) | ||
| Acute phase | ||
| Procedural analgesics | Background analgesics | Anxiolytics |
| Morphine (IVB, IVCI, PCA); Roxanol (oral morphine) | Morphine (IVCI, PCA) | Diazepam (PO) (Valium) |
| Meperidine (IVB, IM) | Meperidine (IVCI, PCA) | Lorazepam (PO) (Ativan) |
| Fentanyl (IVB, IM) | Methadone (PO, NPC) | Alprazolam (PO) (Xanax) |
| Hydromorphone (PO) (Dilaudid) | Sustained release morphine (PO, NPC) (MS Contin) | |
| Nalbuphine (IVB) (Nubain) | Acetaminophen (PO, NPC) | |
| Ketamine (IV, IM) (Ketalar) | NSAIDs (PO, NPC) | |
| Oxycodone (PO) (Percocet) | Choline magnesium trisalicylate | |
| Rehabilitative phase | ||
| For severe pain | For mild to moderate pain | Anxiolytics |
| Hydromorphone (PO) (Dilaudid) | Oxycodone (PO) (Percocet) | Diazepam (PO) (Valium) |
| Fentanyl (transmucosal) | Non-steroidal anti-inflammatory drugs (NSAIDs) with or without narcotics | Lorazepam (PO) (Ativan) |
| Morphine (IVB, IVCI, PCA) | Usually not necessary: Acetaminophen NSAIDs | Alprazolam (PO) (Xanax) |
Many patients also require anxiolytic medication along with the analgesic medication. Therefore, included in Table 64.3 are the anxiolytic agents which are suggested for use by several authors. In a recent review of the practices of three pediatric burn hospitals it is clear that the vast majority of children with moderate and large burns receive both opiates and benzodiazepines.72 This is also the experience in the Shriners Hospital for Children in Galveston Texas.2
Emergency phase
If the initial burn injury can be closed surgically, that gives the best relief of pain. The pain is predominantly related to the open wound. Once the wound is closed, the pain subsides. The use of resection and grafting of open burn wounds significantly reduces the burn pain. Open wounds should be grafted as soon as they are clean enough to do so. Even temporary coverage with cadaver skin or pigskin reduces pain in the area of the burn. In the case of second-degree wounds, the use of BiobraneRX, OpsiteRX, TegadermRX DuodermRX or other wound-covering dressings almost immediately eliminates pain in the burn wound site.73–77 Cultured allogeneic keratinocyte sheets accelerated healing and thereby reduced pain and suffering compared to OpsiteRX treatment.78
Sometimes the pain can be managed by topical agents. Aloe vera has been used as a home remedy for many generations. Recently, there have been several studies to examine its efficacy more thoroughly. Maenthaisong et al. did a systematic review with a meta-analysis and concluded from the published studies of first- and second-degree burns that aloe vera was more effective than the control.79 It was associated with a shorter healing time by 8.79 days. A more recent randomized controlled study in 2009 by Khorasani et al. confirmed the greater efficacy of aloe over silver sulfadiazine.80
As noted under above, physical coverage of the burn wound decreases pain.81,82 For instance, leaving blisters intact leads to less pain.83 But this practice is questionable because of possible infection associated with the blisters. One additional issue concerning procedures is the amount of pain created from removing a dressing over the burned area. These removals are usually facilitated by soaking the dressings off but the soaks are sometimes painful. Some of the newer dressings are easy and painless to remove; moist exposed burn ointment dressings84 are being developed to reduce that pain.
Acticoat was found to be much less painful than silver sulfadiazine in the treatment of partial thickness burns by Varas et al.85 Several new lipidocolloid dressings seem to show promise for reducing pain86,87 Cellulose dressings also reduce pain.88 Several new products are adherent to dry skin but not moist skin and therefore cause much less pain.89,90 Suprathel is a reabsorbable skin substitute which goes even further in reducing the pain of dressing.91
One more novel technique of painlessly cleaning the burn wound is to use an ultrasound mist. There are two short reports advocating this.92,93
Sometimes nerve blocks, either local or regional, work; they are usually done with lidocaine or related compounds. Pedersen et al.94 reported the use of EMLA cream, a prilocaine and lidocaine mixture, to burn wounds in a double-blind randomized manner for 8 h to reduce pain but it did not reduce late hyperalgesia. Whenever EMLA cream is applied to disrupted skin or large areas, caution should be taken to avoid toxicity from systemic absorption. Seizures and other CNS toxicity have been reported from these circumstances.95–97
Both bupivacaine and lidocaine infiltration of the burn wound eschar site or the grafting site have been successfully used to reduce pain.98–100 In non-randomized and non-controlled studies, sympathetic nerve blocks with lidocaine are effective for blocking thermal pain.101–106
Physiological changes and treatment occur in patients with burns >10% total body surface area (TBSA). During the emergency phase, the preferred route for most medications is the intravenous route because of potential problems with absorption from the intramuscular site and stomach due to decreased perfusion. Of the agents recommended for the relief of procedural pain, morphine is the most widely used. For procedures, IVB and IVCI are the most common methods of administration used. For extremely painful procedures in both the emergency and acute phase, fentanyl has a major advantage, in that it is shorter acting and avoids over sedation following a procedure, as might occur with repeated doses of morphine. Higher opiate doses during the initial resuscitations are associated with higher resuscitation volumes107; this is probably secondary to the vascular effects of the opiates. As noted above, we have few studies of the pharmacokinetics of opioids and anti-anxiety drugs in burned patients. Martyn, in a review of pharmacologic studies in burned patients, describes a variety of pathophysiologic changes accompanying burn injury, which can alter drug deposition.108 These changes include cardiovascular changes, alteration in renal and hepatic function, and fluctuations in plasma protein concentration, which may render pharmacokinetic studies of non-burned patients not applicable to burned patients. Since it is difficult to predict precisely how drug responses will be altered, it is important to monitor responses on drug levels more closely so that dosage can be titrated to the individual patient’s needs. In addition to opioid analgesics, anesthetic agents such as ketamine and nitrous oxide may be used for procedural pain and are discussed in more detail below.
Acute phase: background pain and anxiety
Important caveats for the use of PCA should include:
1 An initial bolus in adults of 0.1 mg/kg of morphine or equivalent dose of other drugs
2 Increasing the patient-controlled dose as needed to achieve pain relief (recognizing that tolerance develops at varying rates in individual patients)
3 Planning for a change in dosing regimens at night to include:
Opioid analgesics have been the mainstay of burn pain drug therapy.109 Among the opioids, morphine is the most commonly used for background pain. Morphine can be given orally or parenterally but bioavailability of oral doses is reduced by first pass hepatic metabolism and the dose must be two- to three-fold higher than the IV dose. For procedural pain, other opioids with more rapid onset and shorter duration may be more appropriate for procedural pain.110,111 Treatment with narcotics has been associated with tolerance and a hyperalgesic state that makes pain more difficult to treat.
Of these agents, morphine has continued to be the mainstay for both background and procedural pain management. Long-term use of morphine has several inherent problems. Morphine binds to more than one receptor class. Although the main effect is on the µ-opiate receptor which alleviates pain, it also stimulates the N-methyl-D-aspartate (NMDA) receptor, which causes the development of a hyperalgesic state associated with morphine tolerance and complications such as bowel obstruction. Changing to other opiates, such as methadone, or other compounds, such as ketamine, which block the NMDA receptor pathways can improve the management of pain no longer controlled with morphine.112–116 Other opiates such as hydromorphone (Dilaudid), levorphanol (Levo-Dromoran), fentanyl, and methadone are all excellent oral agents for the relief of moderate to severe pain when given in equianalgesic doses to morphine. Fentanyl comes in a convenient flavored Oralet, especially attractive for use with children. The combination of lorazepam, given with hydromorphone 45–60 min before a procedure, results in reasonable pain control for the majority of adult patients. As with other opioid analgesics, tolerance may develop rapidly, so the dose of hydromorphone may need to be adjusted frequently. Opiate clearance seems to be faster in individuals with burn injury.117–119 Methadone has a longer half-life than morphine and therefore can provide coverage for a longer time between doses. Also methadone has been noted to give smoother pain relief for children postoperatively than morphine.115 Methadone is different from morphine both pharmacokinetically and pharmacodynamically and has been reported to be effective in patients tolerant to and poorly-controlled by morphine. In addition, methadone is a fraction of the cost of most other opioid analgesics.116 We do not use meperidine because many individuals report a rush with IV meperidine, thereby adding to its addictive qualities.
Pain associated with dressing change is very important to manage well. Finn suggested adding patient PCA intranasal fentanyl versus oral morphine.120 In a randomized double blind placebo controlled study of 26 adults, they concluded that intranasal fentanyl was as effective and safe as oral morphine. Also Prakash et al. reported using PCA with fentanyl as an effective alternative.110
From the published literature concerning the use of opioids in patients with burn injury, one would have difficulty making decisions about the best drug, the best dosage range, or the best route or method (i.e. PCA, or continuous infusion) of administration of these drugs.110,117–123 Morphine or other opioid analgesics are considered mainstays of pain relief for burned patients, but many reviewers report less than adequate pain relief for burned patients with the use of such agents.124,125 The line between enough opioid analgesics to provide pain relief and too much causing respiratory depression is difficult to find. Also, the experience of the authors has shown that even a PCA regimen must be individualized and frequently adjusted.
More recently, gabapentin has been advocated for acute burn pain.126 It may be effective because the nature of a burn injury is destruction of nerve endings in the skin. Studies by Cuignet et al. documented that if 2400 mg of gabapentin is given to adult burn survivors per day, the amount of morphine needed is less and the pain control better.127
Anxiety medication should be considered only after the patient’s pain has been aggressively treated. The medical staff can inappropriately attribute the patient’s complaint to anxiety (‘just anxious’ is a frequently used phrase) when, in fact, the patient is really experiencing pain. For anxiety during the acute phase, lorazepam is usually used. Martyn et al.128 studied the pharmacokinetics of lorazepam in burned patients. After a single dose, there was a rapid decline in concentration due to the high lipid solubility and rapid tissue uptake, leading to a shorter hypnotic effect of the drug. Patterson et al.129 reported that, in a double-blind placebo-controlled study of 79 patients, 1 mg lorazepam did significantly reduce procedural pain ratings in those patients with high baseline pain, but did not reduce baseline trait anxiety. Based on Martyn’s work, we would conclude that lorazepam would be superior to diazepam in treating anxiety in burned patients.128 Martyn et al. suggest that, in addition to the fact that the clearance of lorazepam is faster than diazepam in burned patients. In a recent survey of the use of anti-anxiety drugs in burned children, 72% received lorazepam at a dose of 0.03–0.05 mg/kg or higher every 4 h for much of their initial hospitalization.2 Lorazepam provided aid in anxiety control with essentially no side-effects. Fewer than 1% of the children had hallucinations or delirium associated with rapid changes in dose. The occasional child responds with increased agitation or delirium rather than sedation, an occurrence we have also noticed infrequently.130
Occasionally, burn patients are poorly-controlled with morphine even in conjunction with lorazepam. Clonidine or dexmedetomidine have been found effective in such clinical situations and provided suitable sedation and dramatic reductions in morphine dose.131,132 Alpha-2 adrenergic agonists (clonidine and dexmedetomidine) have sedative, anxiolytic, analgesic, and sympatholytic properties. Noradrenergic pathways within the locus ceruleus of the brain stem help regulate vigilance, wakefulness, and sleep. The locus ceruleus contains one of the highest densities of α-2 adrenergic receptors in the body. Administration of α-2 adrenergic agonists produces decreased brainstem norepinephrine turnover resulting in increased activity of inhibitory (GABA) neurons which mediates the sedative and anxiolytic effect of these drugs.133,134 Alpha-2 adrenergic agonists are potent anxiolytics and have been used effectively to treat post-traumatic stress syndrome. Clonidine is also used in controlling attention deficit disorder. Alpha-2 adrenergic agonists can produce profound analgesia through central mechanisms.135 Clonidine and dexmedetomidine have both been found to reduce anesthetic and postoperative analgesic requirements.136,137 Intranasal dexmedetomidine (2 µg/kg) has been found to be equally effective as oral midazolam (0.5 mg/kg) for preoperative sedation of pediatric patients scheduled for burn reconstructive surgery.138 Dexmedetomidine has been found effective as the sole analgesic in ventilated postoperative patients.139,140 Orally administered dexmedetomidine (2.5 µg/kg given 20–30 min pre-procedure) produced suitable sedation and allowed placement of peripheral venous catheters in pediatric patients with neurobehavioral problems.141 Clonidine is useful in pain and anxiety management either alone or in conjunction with opioids.142–144 It has the advantage of not causing pruritus or respiratory depression. Much data exists concerning its use in adults and children in the preoperative and postoperative setting.145,146 Dexmedetomidine has been used to facilitate withdrawal of opioids in critically ill patients.147 When administered in higher doses, dexmedetomidine has been used by itself as a general anesthetic.148
These drugs are distinguished by several unique features. Profound sedation is possible with minimal effects on respiration.149 Patients sedated with dexmedetomidine can often be aroused. The α-2 adrenergic agonists are not controlled substances and there are no reports of substance abuse with clonidine or dexmedetomidine. Use in the ICU has not been associated with withdrawal symptoms even when dexmedetomidine was infused for up to 168 h at 0.21–0.49 µg/kg per hour.149 Long-term administration of dexmedetomidine infusions in burn patients achieved adequate sedation.148,150,149 No tachyphylaxis and no rebound hypertension or tachycardia were observed when the drug was discontinued.
Acute phase: procedural pain and anxiety
During the course of the acute phase of injury, burn patients must endure numerous painful procedures that produce intense physical and psychic stress. Among others, these include initial wound debridement, daily dressing changes, range of motion and exercise therapy, wound staple removal, and placement of intravascular catheters. For a number of reasons, adequate control of the pain and anxiety associated with these procedures is especially challenging (Box 64.2) Poorly-controlled pain can make it difficult to accomplish a procedure effectively or safely and increases anticipatory anxiety that can impair patient compliance and may contribute to behavioral morbidity such as post traumatic stress syndrome.151 In addition, increased sympathetic tone which is associated with exaggerated catabolism, impaired wound healing and immune function are associated with pain and can contribute to prolonged hospitalization.152,153 Effective pain control in adults can often be sufficient to reduce anxiety. In pediatric patients, however, anxiolysis is also needed. For especially stressful procedures, most pediatric patients must be rendered insensible. For this reason, the popular term ‘conscious sedation’ is usually inaccurate and misleading when treating pediatric patients. A very young patient who is aware of the procedure will resist enough to make the procedure difficult or less safe and the anxiety associated with this experience makes future procedures more difficult. The level of sedation and analgesia required in this situation cannot accurately be described as ‘conscious sedation.’ A more accurate descriptive term is moderate or deep sedation for procedures. The depth of sedation must be individualized depending on the intensity of the pain associated with the procedure and the patient’s maturity, level of anxiety, and pain tolerance.