Fig. 8.1
Recurrent nasal polyposis (P) in the right nasal cavity in a patient with AERD and CRS. Septal perforation (s) secondary to previous surgery
Postoperative care for this subset of patients is a controversial topic. Many otolaryngologists utilize high-dose topical steroid irrigations in controlling the recurrence of this disease. However, one study has demonstrated that budesonide nasal irrigations have not been shown to alter postoperative recurrence of the disease at 1 year [7]. More research is needed to establish what effect, if any, high-dose nasal steroid irrigations have on disease progression and the need for revision surgery.
More recently, ASA desensitization has shown promise for patients with CRS and AERD. Aspirin desensitization and daily aspirin maintenance have been established as beneficial in the management of CRSwNP in patients with AERD [5, 8]. ASA desensitization has been shown to have a positive impact on endoscopic polyp scores and is associated with both a decreased frequency of sinus surgeries and sinus infections [5]. The process of ASA desensitization is conducted as an inpatient, medical day unit or outpatient setting. Patients undergo a pretreatment regimen including optimization of pulmonary status, daily montelukast, and treatment of other concomitant conditions. ESS if needed should be timed 4–6 weeks prior to desensitization, as the therapy is more effective at preventing the regrowth of polyps than at reducing polyp size. A challenge is given by increasing doses of ASA until a target dose (usually 325 mg) is reached. The patient is then maintained on a maintenance dose (650 mg twice a day) indefinitely or risk re-sensitization. This dose can then often be weaned after a month if there is adequate symptom response, and systemic corticosteroids are reduced or eliminated [8].
Overall, ASA desensitization and ongoing maintenance therapy is tolerated in the majority of patients with only 8–23 % of the patients experiencing mild adverse events. Side effects including gastritis, dyspepsia, or epistaxis can be barriers to success. ASA desensitization has also shown a significant reduction of oral corticosteroid use by AERD patients and a significant improvement in subjective symptoms; however, double-blind randomized placebo-controlled studies are still necessary to prove causation [5].
Postoperative management in AERD and CRS should include twice-daily nasal saline irrigations with the addition of high-dose topical steroids at the discretion of the clinician. ASA desensitization should be considered in all AERD patients with severe or intractable symptoms or aggressive nasal polyp formation.
Gastroesophageal Reflux (GERD)
GERD has not been shown to cause or at least contribute to CRS. It is known to coexist in nearly half of the patients with postnasal drainage as a complaint, which can be misinterpreted by both patients and general practitioners as ongoing sinus disease [9]. However, GERD has been shown to be more prevalent in patients with refractive CRS than in patients with treatment responsive CRS and in healthy controls [10]. In addition, a few small studies have shown modest improvement of CRS symptoms in patients on once- or twice-daily proton pump inhibitor (PPI) therapy [10]. This suggests a possible causative effect that many feel represents contribution of reflux to underlying inflammation in the sinonasal tract. As such, in the setting of known GERD, symptoms suggestive of GERD, and/or findings on physical exam suggestive of GERD (flexible laryngoscopy findings), a referral for pH probe testing or initiation of PPI therapy should be considered.
Mucociliary Dysfunction
Most inherited forms of ciliary dysfunction, including Kartagener syndrome and cystic fibrosis (CF), are typically diagnosed in childhood. A detailed history and physical exam including past medical history and family history may heighten suspicion for these syndromes in patients with recalcitrant CRS. In the event of elevated suspicion, further testing for evaluation of Kartagener syndrome or CF should be considered. A referral to genetics for further genetic testing and counseling is necessary for any positive or equivocal test results.
Kartagener syndrome is a primary form of ciliary dyskinesia due to an abnormality in the dynein arm and is diagnosed by the saccharine mucociliary transport test or nasal biopsy with electron microscopy. It is associated with recurrent lung, ear, and sinonasal infections in children as well as hyposmia, infertility, and the findings of situs inversus and dextrocardia.
CF, an autosomal recessively inherited disease, results in secondary ciliary dysmotility by altering the viscosity of mucous through the disruption of transmembrane transport of chloride ions. The body’s mucociliary transport mechanisms are not efficient with this more viscous form of mucous. Recurrent Pseudomonas aeruginosa and Staphylococcus aureus colonization and infections are common. CF is typically diagnosed in childhood in the setting of bronchiectasis, recurrent pulmonary infections, CRS, malabsorption, and stunted growth. CF should be ruled out in any child that presents with nasal polyps. Diagnosis is made through newborn screening, sweat testing, and/or genetic testing. CT imaging in CF will often reveal hypoplastic sinus cavities with mucosal thickening and sclerosis and thickening of the adjacent bony framework (Fig. 8.2). Some less severe phenotypes of CF may not be diagnosed until adulthood and should be in the differential diagnosis in refractory CRS.
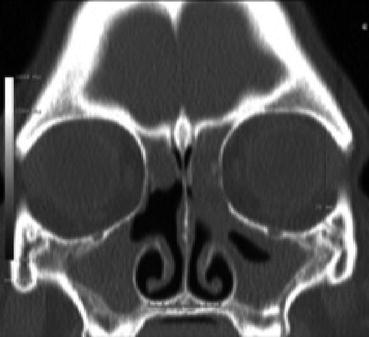

Fig. 8.2
Mucosal thickening and opacification of the bilateral maxillary, frontal, and ethmoid sinuses with characteristic hypoplastic maxillary sinuses as seen in CF
The management of CF is difficult and requires a multidisciplinary approach. As the pathophysiology of this disease results in a chronic process, the management of CRS is primarily medical. However, many patients fail medical management and require a surgical approach. In general, the indications for ESS are sinus disease that is contributing to pulmonary exacerbations and declining pulmonary function, medically refractory polyposis with nasal obstruction, and lung transplant candidacy [11, 12]. As the survival of cystic fibrosis patients continues to increase, this number will likely increase. Larger surgical openings are generally advocated for the refractory CF patient with significant CRS.
Biofilms
Certain common bacterial species including Staphylococcus aureus and Pseudomonas aeruginosa are capable of forming biofilms. Bacterial biofilms are defined as an assemblage of microbial cells enclosed in a self-produced polymeric matrix that is irreversibly associated with an inert or living surface [13]. The organized communities of bacteria attached to the sinonasal mucosa can then release planktonic bacteria that create acute exacerbations. The adherent and organized nature of the biofilm imparts a resistance to standard oral antibiotics.
The confirmation of the presence of biofilms depends on identification by scanning electron microscopy, confocal laser microscopy, or transmission electron microscopy, which are not accessible in the clinical setting. As such, the diagnosis is more often made with positive cultures for typical biofilm forming species in the setting of recalcitrant disease. CRS patients with biofilms have been shown to have more severe disease both preoperatively and postoperatively suggesting a role in recalcitrant disease [14].
Studies looking at topical antibiotic therapies have shown mixed results in patients with CRS. Various surfactants including 1 % baby shampoo in normal saline and manuka honey have shown some promising results in overall symptom control and antibiofilm activity; however, more research is necessary to establish evidence-based recommendations [15].
Immunodeficiency
Patients with immunodeficiency have recalcitrant disease because of their underlying immune disorder. Recurrent sinopulmonary infections are the most prevalent infections among primary immunodeficiency patients. CRS can be seen in common variable immunodeficiency (CVID), selective IgA deficiency, IgG subclass deficiency, and specific polysaccharide antibody deficiency [16]. CVID is the most common symptomatic primary immunodeficiency in adults and has been observed in up to 10 % of patients with refractory CRS [16, 17]. Further, more than 20 % of patients with CRS have lower than normal levels of one or more immunoglobulins [18].
Evaluation for immunodeficiency should be considered in patients with frequently recurrent, persistent, and/or severe infections or recalcitrant rhinosinusitis with rare organisms. These patients may also have associated atopy, autoimmune disease, or gastrointestinal disease. The importance of identifying an underlying immunodeficiency cannot be stressed enough as the management changes drastically. More judicious use of antibiotics, both prophylactic and culture directed, should be used, and IVIG may be indicated in certain situations. Once identified, these patients should be monitored in coordination with an immunologist.
Revision Surgery
As mentioned, sometimes more surgery can offer an advantage in the management of the refractory CRS patient who is not doing well after a prior surgical intervention. Both the endoscopic exam and repeat CT imaging will characterize when the etiology for failure is anatomic. The decision to proceed with revision surgery should be made on an individual basis depending on the underlying contributing factors. Symptomatic patients with obstruction on imaging or symptomatic patients with a significant disease burden are likely to be good candidates. The role of revision surgery in CRS is to improve medical management by reducing disease load and improving access for irrigations and topical therapies.
It has been estimated that 8–20 % of patients undergoing ESS will require revision surgery [19, 20]. Multiple studies have reviewed the common anatomic reasons for revision surgery. Musy reported the most common anatomic factors leading to revision surgery were lateralization of the middle turbinate (78 %), incomplete anterior ethmoidectomy (64 %), scarred frontal recess (50 %), incomplete posterior ethmoidectomy (41 %), and middle meatal antrostomy stenosis (39 %) [21]. Gore et al. noted residual anterior and posterior ethmoid cells or septations (75 %), a residual uncinate process (64 %), residual agger nasi cells (64 %), unopened sphenoid sinuses (53 %), and frontal cells (45 %) on preoperative imaging of patients undergoing revision surgery [22]. Bassiouni identified the most common location for polyp recurrence to be in the frontal sinus or frontal recess (55 %) followed by the ethmoid cavity (38 %) [23].
Revision surgery of the maxillary sinus is most often required when there is a residual uncinate process or a surgically created opening into the sinus not confluent with the natural ostium. The latter predisposes to recirculation of mucus from the natural opening back into the sinus via the “false” surgical opening, predisposing the patient to recurrent infections. A remnant uncinate can result in a bridge of tissue between the natural os and the surgical antrostomy causing recirculation (Fig. 8.3). Recirculation can also occur secondary to scarring or incomplete initial antrostomy. Another cause of maxillary sinus obstruction is persistent infraorbital ethmoid cells (Haller cells). These cells can be overlooked due to a more anterior position than expected. A 30° scope can be utilized to both ensure the natural os is included in the antrostomy and that there is not a residual Haller cell.
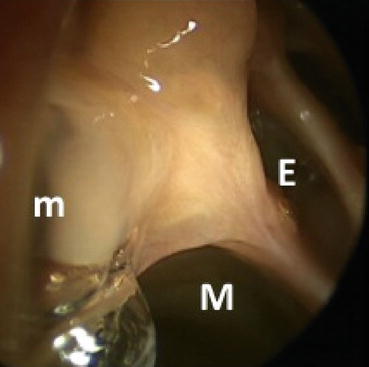

Fig. 8.3
Recirculation of mucous from the natural maxillary os (m) on the right into the maxillary antrostomy (M). Adjacent ethmoid (E) cells are also visible
Although total ethmoidectomy is not indicated in all patients undergoing primary endoscopic sinus surgery, most will undergo uncinectomy, anterior ethmoidectomy, and maxillary antrostomy if they have severe enough disease to necessitate surgery. In revision ethmoid surgery, the entire ethmoid labyrinth should be opened in a posterior to anterior fashion. Surgical navigation can be helpful to identify unopened cells and the location of the skull base and lamina papyracea. The strut of the horizontal segment of the basal lamella should remain intact to prevent lateralization of the middle turbinate. A curette should be used to ensure the medial bulla has been removed, and all of the bony partitions along the lamina should be removed to reduce the mucosal surface area for recurrent polyp growth. Mucosal preservation will reduce scarring and antrostomy stenosis; therefore, sharp dissection should be used to prevent mucosal stripping.
Patients with chronic frontal sinusitis should at minimum undergo resection of the agger nasi cell and complete excision of the superior uncinate process. Preoperative analysis of the frontal recess anatomy on imaging (especially using sagittal CT reconstructions) is particularly important to identify the drainage pathway and to identify reasons for failure. If the frontal recess is not obvious intraoperatively, an image-guided probe can be invaluable. Once identified, synechiae and bone fragments can be cut and removed. Curettes can be used to take down the beak anteriorly. A lateralized middle turbinate (Fig. 8.4) can often be an iatrogenic cause for frontal sinusitis; this can be addressed by medialization with a stitch (pexy), spacer, or middle turbinate resection. The frontal sinus rescue procedure has also been described as a method to prevent recurrent stenosis [24]. More recently, aggressive management of the frontal recess by Draf 3/modified Lothrop/frontal drillout procedure has been shown to reduce long-term (>12 months) polyp recurrence, especially in more complicated patients with asthma and AERD [23].
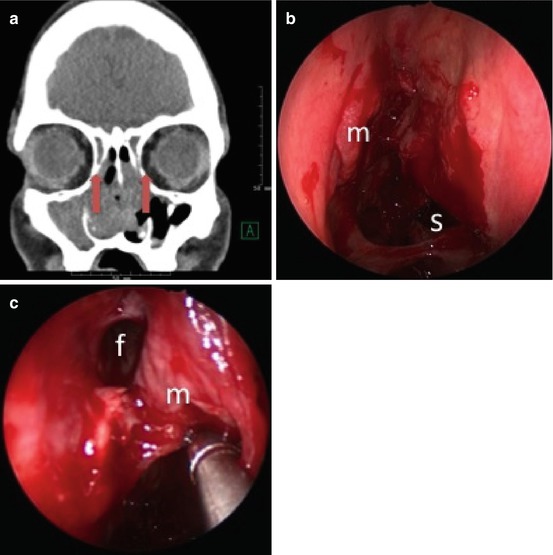

Fig. 8.4
(a) Lateralized middle turbinate remnant (arrows) obstructing the frontal recess bilaterally in the setting of AERD with recurrent polyps. (b) Lateralized middle turbinate remnant (m) obstructing the right frontal sinus. Posterior septal defect (s) and synechiae (arrow) secondary to previous surgery. (c) Right frontal recess (f) after medialization of the middle turbinate (m) and balloon dilation
The sphenoid sinus may be obstructed from stenosis and scarring. The location of the natural os can be identified medial to the superior turbinate or turbinate remnant. Image guidance may be helpful. A large sphenoidotomy should be created in revision surgery to reduce the risk of restenosis and to allow for adequate topical drug delivery. This should be accomplished in a medial and inferior direction using through-cutting instruments and good visualization to avoid vascular injury and to cut through the thick sphenoid bone.
Overall, the goals of revision surgery are to widely open obstructed sinus cavities, decrease disease burden by removing bulky polypoid tissue, remove residual cells and partitions that are acting as a nidus for infection or polyposis, and improve access for irrigations and topical therapies.
Topical Drug Delivery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






