The lymphatic system is interrelated with all of the other systems of the body. Its primary roles include conducting immune system surveillance, assisting the cardiovascular system to maintain fluid homeostasis, and aiding the digestive system in the breakdown of long-chain fatty acids. The immunological functions involve both the immediate response to pathogens and the long-term resistance to repeated exposure to pathogens.
ANATOMY OF THE LYMPH SYSTEM
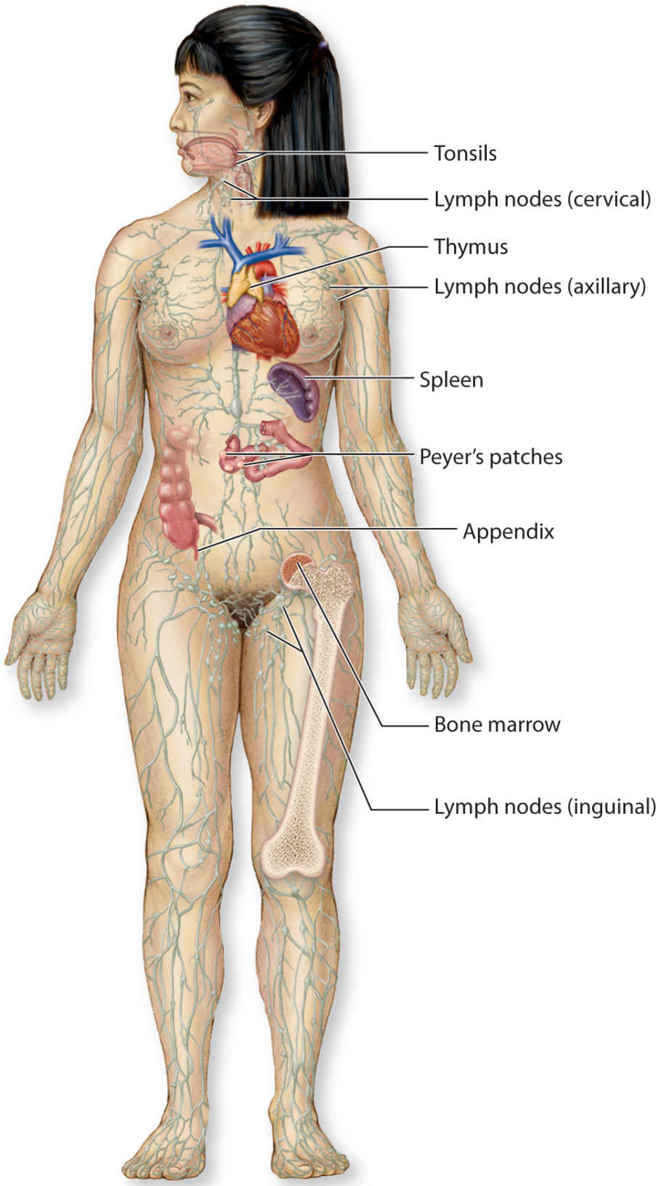
The body’s immunological function is performed by the lymphoid organs, including the spleen, thymus, tonsils, Peyer’s patches located in the small intestine, bone marrow, and lymph nodes. (FIGURE 5-1)
FIGURE 5-1 Anatomy of the immune system
Spleen provides lymphocyte proliferation, immune surveillance, and immune response; removes old or damage platelets and blood cells from the blood; removes debris and foreign matter from the blood. It has other functions not related to the lymph system that are not discussed here.
Thymus secretes thymosin and thymopoietin, hormones that enable T-lymphocytes to become activated against specific pathogens; it does not directly fight the antigens.
Tonsils capture and remove pathogens in the airway during inhalation.
Peyer’s patches, structurally similar to tonsils, are located in small intestines. They destroy bacteria in the intestines and generate “memory lymphocytes” for long-term immunity.
Bone marrow, also called myeloid tissue, is responsible for production of all the cells of the immune system through the process of hemopoiesis. The stem cells become common myeloid stem cells or common lymphoid stem cells that further differentiate into red blood cells, platelets, and white blood cells.
Lymph nodes are the biological filter station for antigens present in lymph fluid; responsible for purifying and draining lymph fluid.
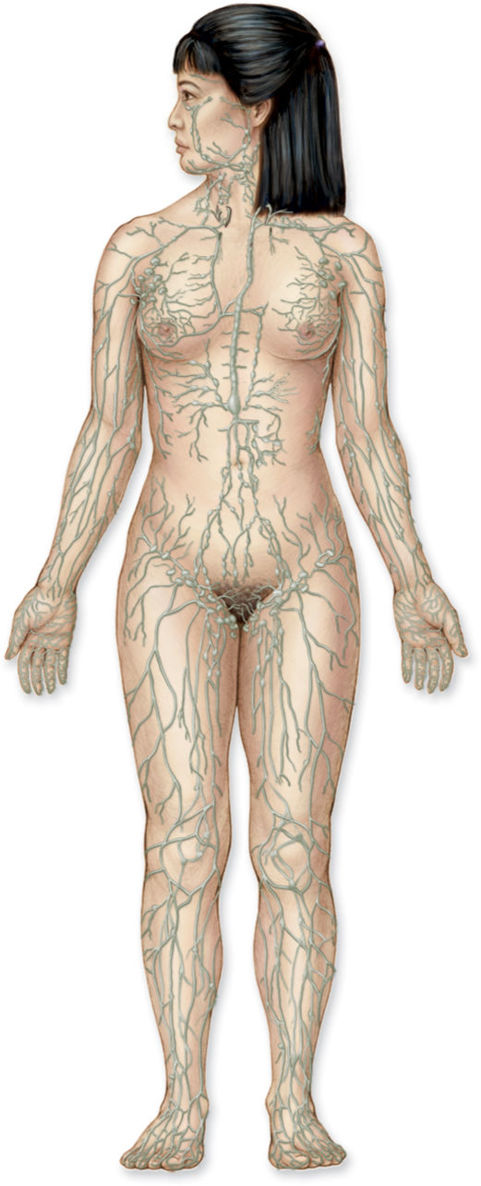
The lymphatic system is a body-wide network of superficial and deep vessels connected by perforating vessels, similar to the venous system. It is comprised of lymph capillaries, lymphatic precollectors, collectors, ducts, and trunks. (FIGURE 5-2) The superficial vessels, located directly under the skin and above the fascia, drain the dermis and subcutaneous tissues. The deep vessels are located below the fascia and drain all the tissue deep to the fascia. The connecting vessels perforate the fascia to transport the fluid between the systems. The lymphatic vessels are similar to arteries in that the larger collecting vessels have smooth muscle in the wall, and they are similar to the veins in that the collecting vessels contain valves that allow for unidirectional flow of the fluids.
FIGURE 5–2 Full-body lymphatic system The superficial network of lymphatic vessels have specific drainage routes to regional lymph nodes.
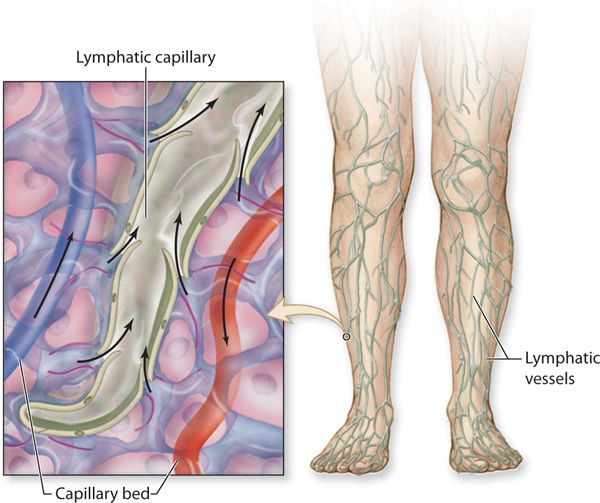
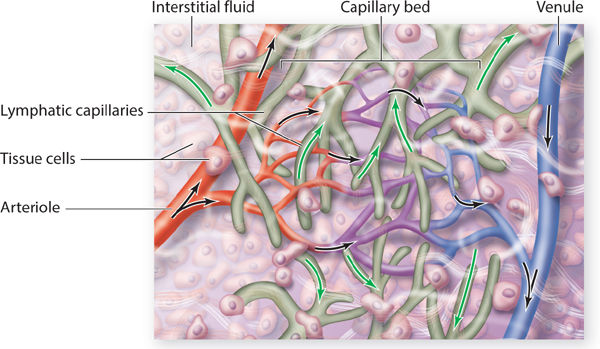
The superficial system begins with the lymphatic capillaries that appear much like a fishnet underneath all of the skin. (FIGURE 5-3) The capillaries are the smallest-diameter vessels and thus have the largest numbers. As the diameter of the more proximal lymphatic vessels increases, the numbers decrease. Lymph capillaries have anchoring filaments that attach to the basement membrane. (FIGURE 5-4) The lymph capillaries are more permeable than the vascular capillaries and therefore can absorb larger molecules of protein and fat. Each lymph capillary is composed of a single layer of overlapping flat endothelial cells, and the overlapping junction is called the inlet junction. The capillary does not contain valves, thus allowing the fluid to move in any direction within the capillary system. The capillaries merge and become precollectors, which have a larger diameter and therefore a lower pressure, thus facilitating movement of the fluid from the capillary to the precollector.
FIGURE 5-3 Lymphatic capillaries that intertwine with the arterial and venous capillary bed Molecules and fluid flow from the arterial capillary bed into the tissue spaces (black arrows) and from the interstitial spaces into the initial lymphatic capillary, termed reabsorption (white arrows). Lymph fluid then flows from the initial lymph capillaries into slightly larger vessels, termed precollectors. Fat and protein molecules that are too large to enter the venous capillaries must be reabsorbed by the initial lymphatics or initial lymph capillaries.
FIGURE 5–4 Anchoring filaments of the lymphatic capillaries The initial lymphatic capillary is connected to the basement membrane by anchoring filaments. When the interstitial pressure gradient (ie, concentration of protein molecules) is high, tension is exerted on the anchoring filaments, which results in pulling the single layer of overlapping endothelial cells. This tension opens the filament and allows the fluid to enter the initial lymphatic capillary where the pressure is low.
The precollectors also have the overlapping endothelial anatomy that allows them to absorb interstitial fluid, and they have a limited number of valves and limited smooth muscle structure that allows them to initiate transportation of the collected fluids. Some of the precollectors of the superficial lymphatic system perforate the fascia and provide a direct connection to the deep system.
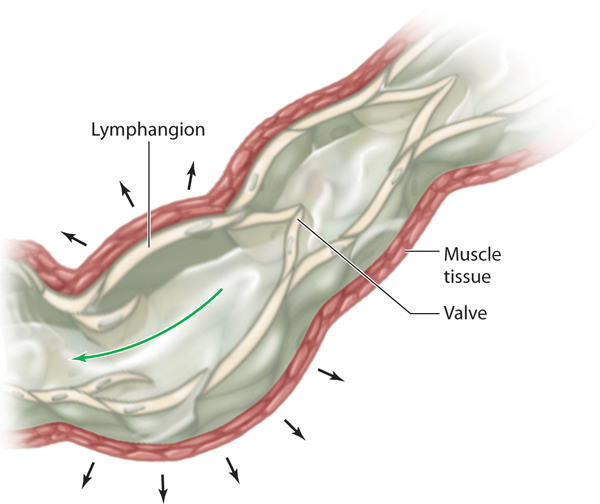
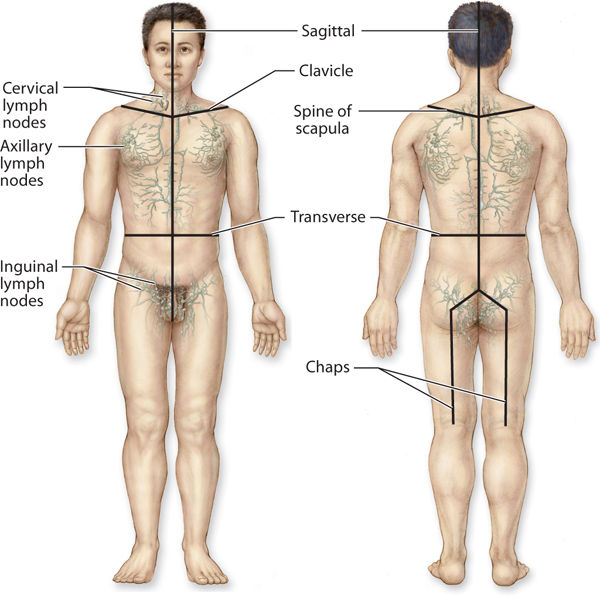
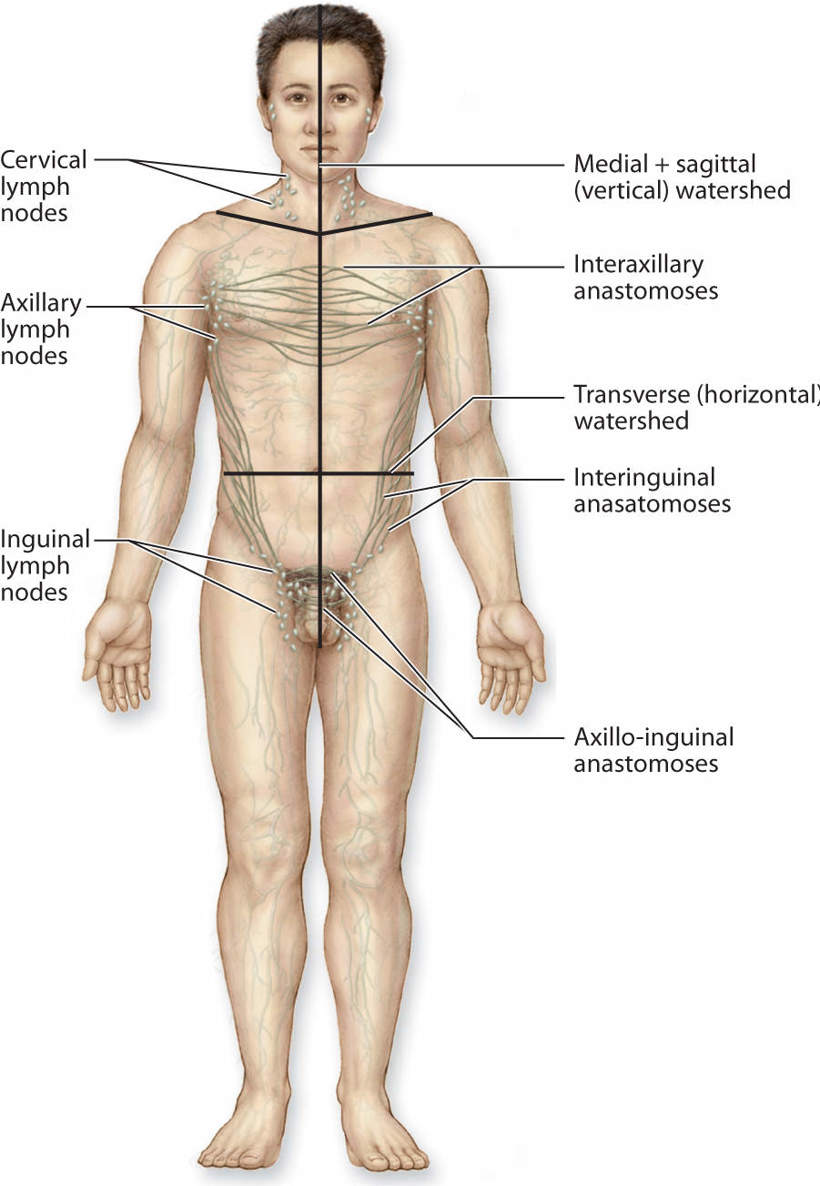
All of the precollectors connect to the next-size vessel, termed collectors. The collectors first bring the lymph fluid to the lymph nodes and then carry the remaining fluid to the larger lymphatic trunks. The anatomical structure of the collectors is similar to the construction of the veins in that they have three distinct layers (intima, media, and adventitia). They have well-defined valves that permit flow to go in only one direction—toward the heart. Within the lymph collector, the vessel segment between two valves is termed the lymphangion (FIGURE 5-5). Each lymphangion has an autonomic-driven resting contraction rate of approximately 10–12 contractions per minute.1 This is further discussed under physiology. The superficial lymphatic collectors are located in the subcutaneous fatty hypodermis and they follow a direct route toward the regional lymph nodes. The deep lymphatic collectors follow the pathway of larger blood vessels. Lymph collectors drain lymph fluid from specific areas of the body, creating lymphatic “territories” that drain to specific regional lymph nodes. These lymphatic territories are defined by lymphatic watersheds that tend to delineate the body into specific collection and drainage patterns. (FIGURE 5-6) Although there is some cross-connection between the territories via the watersheds, most of the fluid flows directly to its own regional lymph nodes. If there is fluid overload in one area, some of the fluid can be diverted to another region via the watershed connections.
FIGURE 5-5 Lymphangion The lymphangion is adjacent to the muscle; thus, when the muscle contracts the lymph flow is increased. Valves and smooth muscle in the angion promote directional flow of the fluid and prevent reflux, much like the valves in veins.
FIGURE 5–6 Lymphatic watersheds The body is divided into lymph territories that are a result of the lymph flow from specific regional lymph nodes to specific body regions. The deep vessels do not cross between watersheds (areas of collection); however, there are some superficial vessels that cross the watershed boundaries and thereby divert lymph fluid from one quadrant to another when there are conditions of overload. The superficial pathways that cross between watersheds can be encouraged with manual lymphatic drainage to direct more flow to open areas; however, it is usually inadequate to remove the majority of the fluid in the affected quadrant.
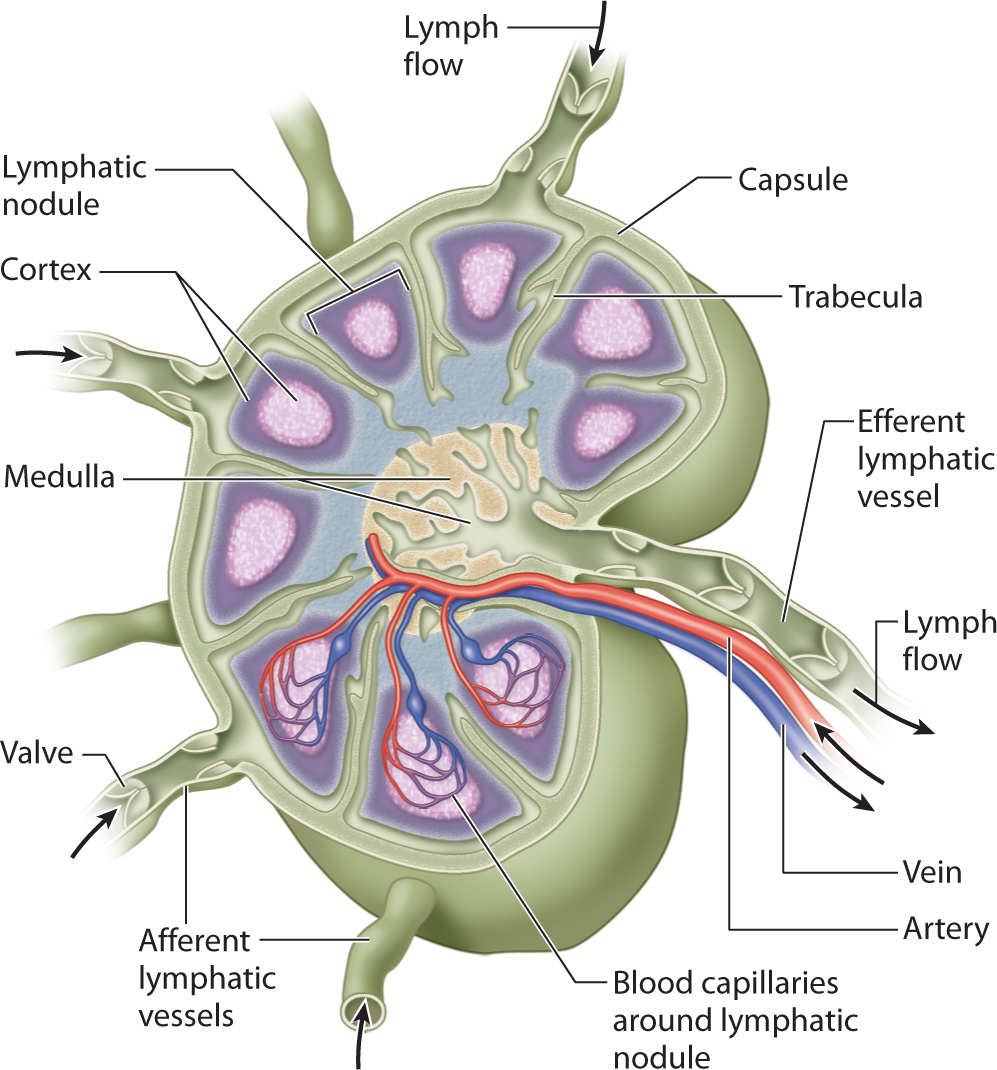
The lymph nodes (estimated to be about 700) are located in clusters throughout the body. (FIGURE 5-7) The lymph node is bean-shaped and surrounded by a dense fibrous capsule. (FIGURE 5-8) The collectors converge at the convex side of the lymph nodes where they are termed afferent lymph vessels. The fluid moves through the cortex of the node, into the medulla, and exits on the concave side via efferent vessels that then become trunks. The number of efferent vessels is less than the number of afferent vessels, resulting in a decreased transportation rate through the lymph nodes, thus allowing the immune system cells time to phagocytose the pathogens that are in the lymphatic fluid.
FIGURE 5-7 Clusters of lymph nodes throughout the body Lymph nodes are located strategically throughout the body, in conjunction with the superficial anastomoses that allow fluid to cross the watershed boundaries. The lymph system naturally diverts fluid across the watershed during times of overload; however, in lymphedema it is insufficient to manage the total volume of fluid needing to be transported. Manual lymphatic mobilization stimulates these anastomoses to increase the rate of flow.
FIGURE 5–8 Lymph node anatomy A greater number of efferent vessels bring fluid to a lymph node and a lesser number of efferent vessels leave the lymph node. This anatomical arrangement permits a slower rate of lymph transport through the lymph nodes and thus allows time for the immune system to phagocytose bacteria, waste products, and dead cells. For individuals with cancer who undergo a lymph node dissection, the rate of flow through the regional lymph node is automatically decreased. This sets the stage for potential lymphatic congestion in the affected quadrant and may lead to lymphedema.
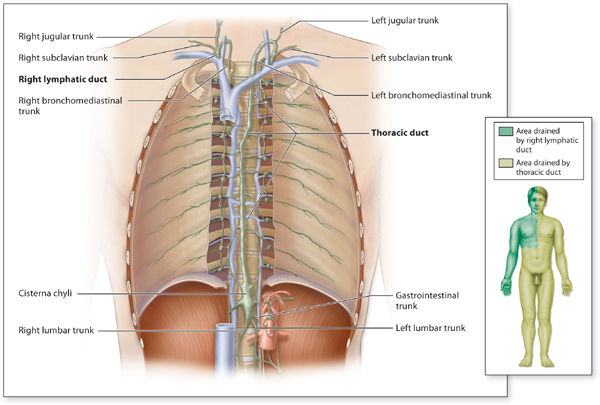
When the superficial and deep collectors converge, the vessels are termed trunks. The trunks are similar in construction to the collectors except they are larger in diameter and contain more smooth muscle in the vessel wall. (FIGURE 5-9) The trunks are named as follows: right and left lumbar trunks, gastrointestinal trunk, jugular trunk, supraclavicular trunk, subclavian trunk, peristernal trunk, and bronchial-mediastinal trunk. The right and left trunks and the gastrointestinal trunk converge in the anterior area of T-11-L2 to form the cisterna chyli, which is the beginning of the thoracic duct. The thoracic duct ascends cephalically along the anterior vertebral column and perforates the diaphragm.
FIGURE 5–9 Lymphatic trunks and the territories that drain into the trunks The collecting vessels merge to form lymphatic trunks.
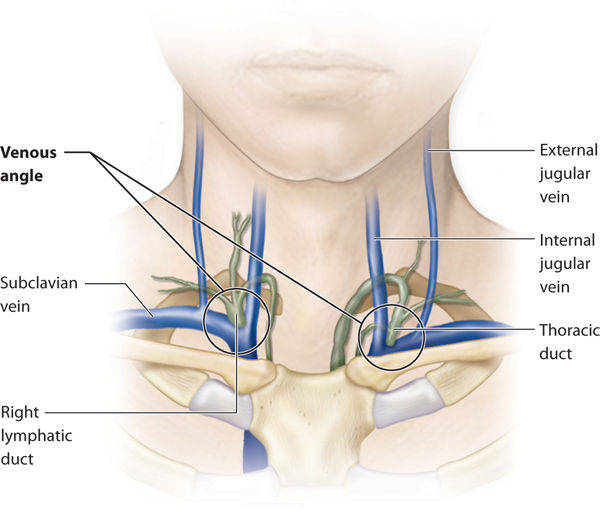
The only direct connection between the lymphatic system and the venous system occurs where the thoracic duct (on the left) and the right lymphatic duct enter the venous angles. The venous angle is formed by the convergence of the internal jugular and subclavian veins. (FIGURE 5-10) This is the anatomical termination of what is defined as the lymphatic system.
FIGURE 5–10 Venous angle The venous angle is formed by the junction of the internal jugular vein and the subclavian vein. The thoracic duct on the left and the right lymphatic duct insert directly into this junction, which is termed the venous angle. The venous angle flows into the brachiocephalic vein.
PHYSIOLOGY OF LYMPHATIC FLOW
The purpose of the lymphatic capillaries is to absorb interstitial fluid and molecules, and when the fluid enters the capillaries, it is termed lymphatic fluid. The endothelial cell junctions overlap, creating inlet valves, so that when the interstitial concentration increases, tension on the anchoring filaments causes the inlet valves to open and allows absorption of the larger fat and protein molecules. These inlet valves remain open as long as the pressure remains higher than the pressure within the lymph capillary.
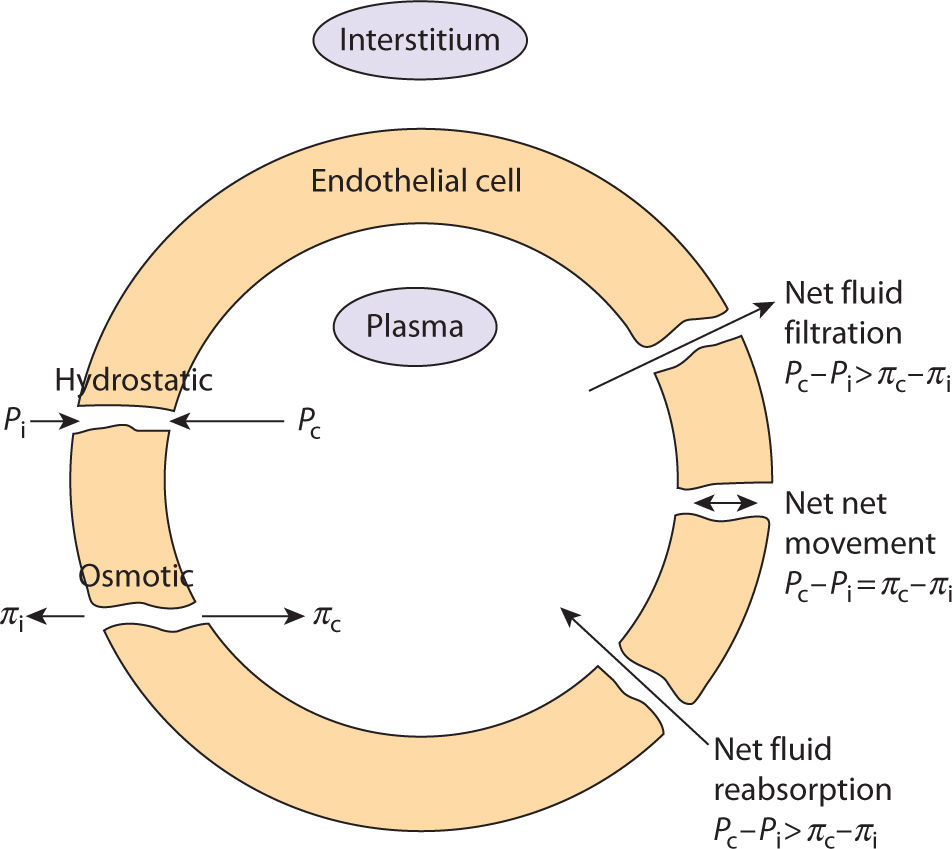
Fluid movement between the capillaries (arterial, venous, and lymphatic) and the interstitial spaces is governed by Starling’s law of equilibrium. This law describes how the osmotic and hydrostatic pressures in the capillaries and interstitial tissue determine the direction of fluid movement. The irony of this equation is that equilibrium is never really achieved because of the dynamic nature of the human body, which does not allow the pressure gradient to equal zero; therefore, fluid is always moving, or in physiologic terms, fluid is constantly being filtrated and reabsorbed at the capillary bed.2,3 (FIGURE 5-12)
FIGURE 5–12 Starling’s Law of Equilibrium
The difference between the net hydrostatic pressure and the net osmotic pressure is the force that determines the direction of fluid flow. (TABLE 5-2) When the fluid flows from the interstitial space into the capillary, it is termed reabsorption and can occur at both the venule and the lymphatic capillary. When the fluid flows from the capillary into the interstitial space, it is termed filtration. Equilibrium occurs when an equal amount of fluid moves from the arterial capillary into the interstitial space and out of the interstitial space into the venous and lymphatic capillaries. Hydrostatic pressure is the pressure exerted by the blood on the capillary walls. Because the pressure is higher on the arterial side than on the venule side, fluid is pushed out of the capillary bed into the interstitial space. Osmotic pressure is the force created by large molecules such as plasma proteins, and it opposes hydrostatic pressure. Plasma protein molecules attract water, so that in a normal system they facilitate reabsorption of the fluids back into the venule.4
TABLE 5-2 Definitions for the Physiological Components of Starling’s Law of Equilibrium

The blood capillary wall is a semipermeable membrane so that a force is required to move the larger protein molecules in and out of the capillary bed. At the arterial end where the net hydrostatic pressure is higher than the net osmotic pressure, the molecules are forced out of the capillary bed and into the interstitium. However, at the venule end, the net osmotic pressure is higher than the net hydrostatic pressure so the pressure is not sufficient to move the molecules from the interstitium into the capillary bed. Therefore, the molecules are reabsorbed into the lymphatic capillary at the overlapping endothelial junction. When this process is in balance so that fluids are moved, no edema occurs. When there is an imbalance, for a variety of reasons and in either direction, edema develops. All edema pathology can be related to a problem with either filtration or reabsorption. When this happens, there is a transportation dysfunction—the freeway is clogged. The challenge to the clinician is to determine the pathology that prevents adequate flow of the fluid back into the central vascular system.
PATHOPHYSIOLOGY OF LYMPHEDEMA
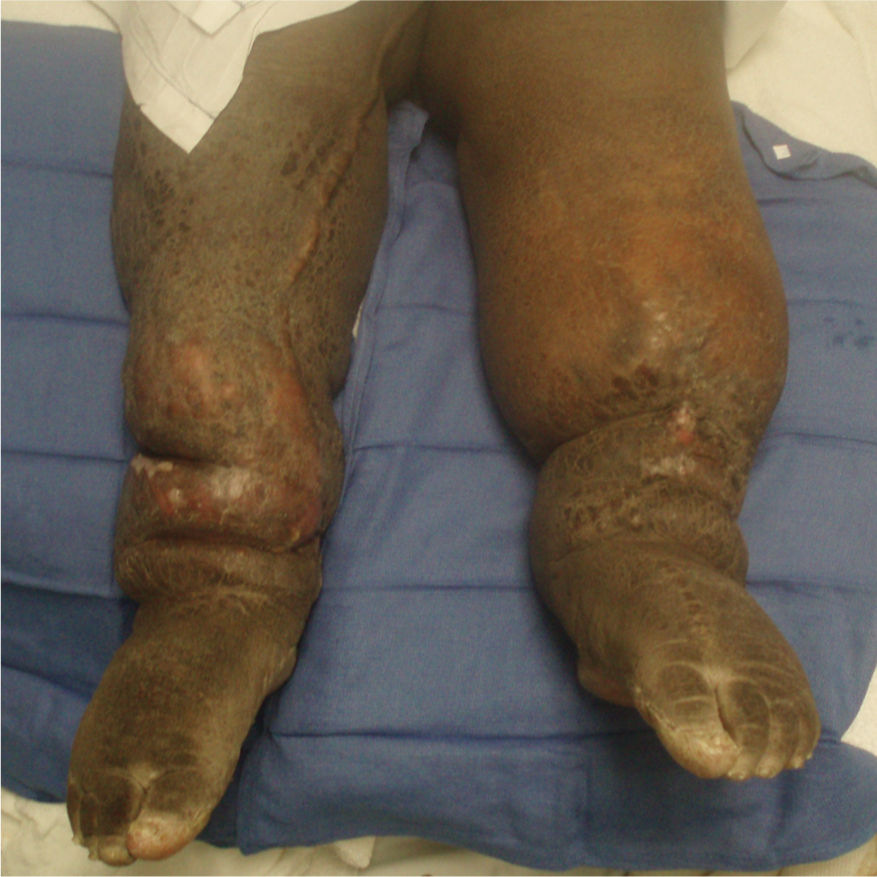
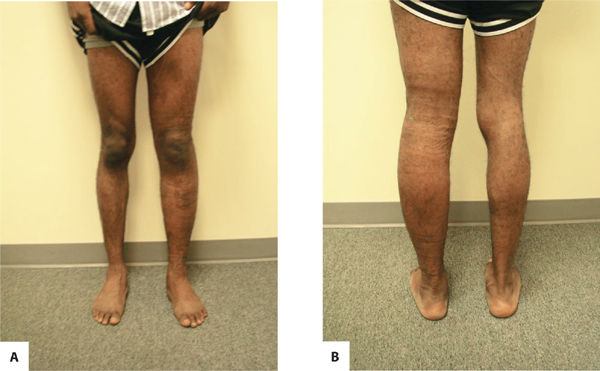
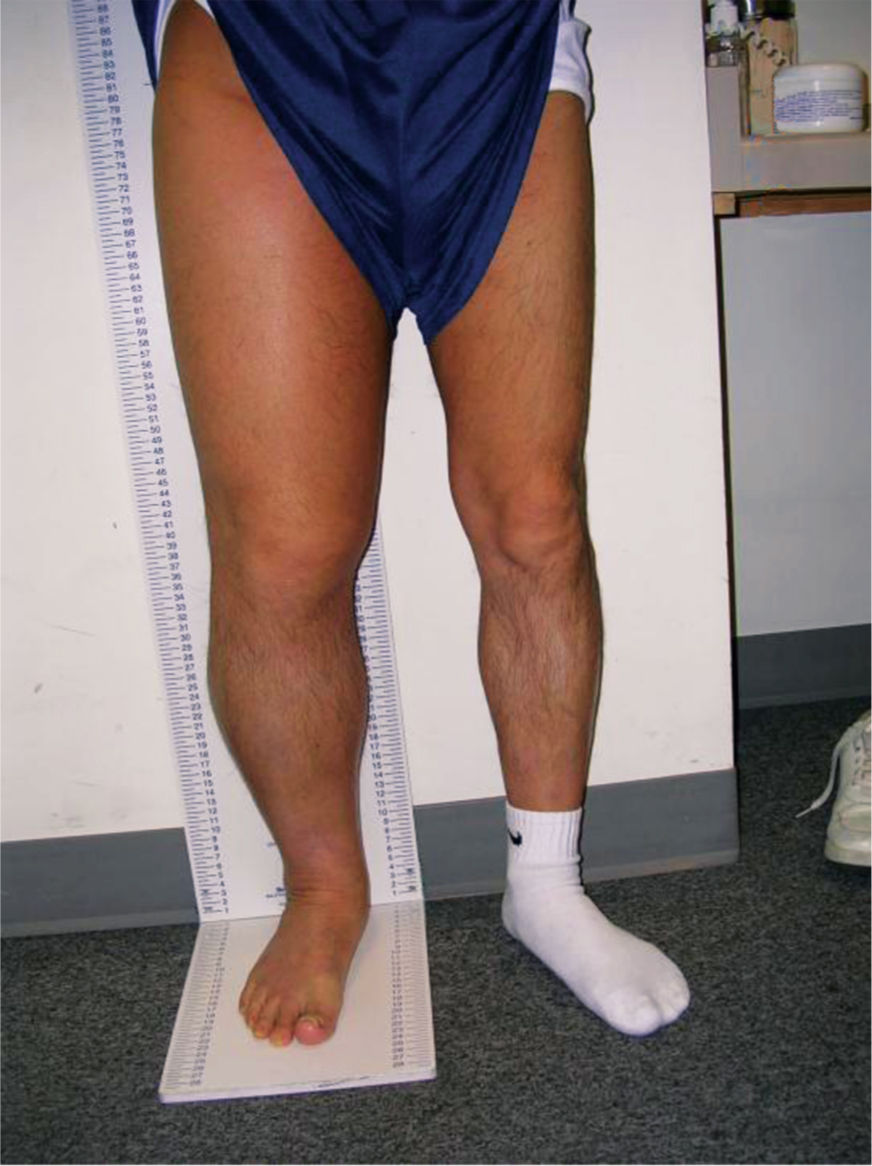
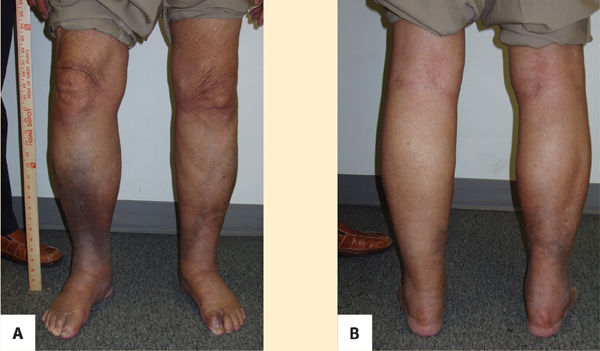
Primary lymphedema is a result of congenital malformations of the lymphatic system, although the consequences may not be observed in the early years. (FIGURES 5-13, 5-14) Secondary lymphedema is a result of acquired damage to the lymph vessels or nodes and therefore impaired reabsorption and/or transportation of lymphatic fluid (FIGURE 5-15). Refer to TABLE 5-1 for more details of both types.
FIGURE 5-13 Primary lymphedema Primary lymphedema in an Advanced Stage II progressing to Stage III; presents with significant skin changes and open wounds on L leg.
FIGURE 5-14 A. Lymphedema praecox (anterior view). B. Lymphedema praecox (posterior view) Anterior and posterior views of Stage II primary lymphedema as a result of cancer and treatment. Note that the contour of the left lower extremity is proportionate even though the size discrepancy is obvious.
FIGURE 5–15 Secondary lymphedema
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Reports a mild heart attack and takes the following medications:
Reports a mild heart attack and takes the following medications: Reports his last ejection fraction was 45%; patient is seen by his cardiologist two times a year.
Reports his last ejection fraction was 45%; patient is seen by his cardiologist two times a year. Reports being careful regarding high-fat, high-cholesterol foods, but admits to liking ice cream daily.
Reports being careful regarding high-fat, high-cholesterol foods, but admits to liking ice cream daily. Works as owner of a moving van company and goes to office daily.
Works as owner of a moving van company and goes to office daily. Uses a rolling walker with a seat in all environments except at work.
Uses a rolling walker with a seat in all environments except at work. Lives with his wife in a one-story house with a daily housekeeper.
Lives with his wife in a one-story house with a daily housekeeper. Unable to sit or stand without upper extremity assist; with UE assist, able to do 3 in 30 seconds.
Unable to sit or stand without upper extremity assist; with UE assist, able to do 3 in 30 seconds. Six-minute walk test with the walker, 390 yards.
Six-minute walk test with the walker, 390 yards. Bilateral trendelenberg gait with the rolling walker, has little or no heel/toe sequence during gait cycle.
Bilateral trendelenberg gait with the rolling walker, has little or no heel/toe sequence during gait cycle. ABI: left is 0.65, right is 0.7.
ABI: left is 0.65, right is 0.7. Pulses: left DP 1+, PT 2+; right DP 2+, PT 2+.
Pulses: left DP 1+, PT 2+; right DP 2+, PT 2+. Auscultation of lungs—negative for crackles or congestion, decreased breath sounds in bilateral lower lobes.
Auscultation of lungs—negative for crackles or congestion, decreased breath sounds in bilateral lower lobes. Auscultation of heart—negative for S3 heart sounds.
Auscultation of heart—negative for S3 heart sounds. Monofilament testing: + response to 5.07 monofilament on both plantar feet, all points.
Monofilament testing: + response to 5.07 monofilament on both plantar feet, all points. Range of motion—hip extension bilaterally to neutral, lacks 5 deg of knee extension bilaterally (5–120 deg knee flexion), 5 deg DF, 20 PF.
Range of motion—hip extension bilaterally to neutral, lacks 5 deg of knee extension bilaterally (5–120 deg knee flexion), 5 deg DF, 20 PF. Strength—gluteus medius bilaterally 3−/5, hip extensors, 3−/5, ankle 2+/5.
Strength—gluteus medius bilaterally 3−/5, hip extensors, 3−/5, ankle 2+/5. Patient reports glucose levels range from 120 to 200 fasting, last HbA1C (per lab results) was 7.5.
Patient reports glucose levels range from 120 to 200 fasting, last HbA1C (per lab results) was 7.5. What are the patient’s risk factors for lymphedema?
What are the patient’s risk factors for lymphedema? What is the physiological rationale for his lymphedema?
What is the physiological rationale for his lymphedema? Is there any other information needed before treatment can be initiated?
Is there any other information needed before treatment can be initiated?