3 Lymphatic reconstruction of the extremities
Synopsis
 In developed countries most cases of lymphedema are caused by a local obstruction of the lymphatic system after iatrogenic intervention.
In developed countries most cases of lymphedema are caused by a local obstruction of the lymphatic system after iatrogenic intervention.
 With the help of advanced microsurgery lymphatic vessels can be sutured and lymphatic bypasses can overcome blocked lymphatic and lymphovascular areas.
With the help of advanced microsurgery lymphatic vessels can be sutured and lymphatic bypasses can overcome blocked lymphatic and lymphovascular areas.
 Microsurgical lymphatic reconstruction is one step in the treatment algorithm. Initially, conventional treatment including physical therapy, exercises designed to mobilize tissue fluid, and compression should be instituted for 6 months. Thereafter the option of a direct reconstruction should be taken into consideration early. Resectional interventions are the very endpoint of the algorithm.
Microsurgical lymphatic reconstruction is one step in the treatment algorithm. Initially, conventional treatment including physical therapy, exercises designed to mobilize tissue fluid, and compression should be instituted for 6 months. Thereafter the option of a direct reconstruction should be taken into consideration early. Resectional interventions are the very endpoint of the algorithm.
 Microsurgical lymphatic grafts have been shown to be effective by independent investigators in nuclear medicine – long-term significant improvement of lymphatic flow – and in radiology – long-term patent lymphatic grafts (more than 10 years) are found.
Microsurgical lymphatic grafts have been shown to be effective by independent investigators in nuclear medicine – long-term significant improvement of lymphatic flow – and in radiology – long-term patent lymphatic grafts (more than 10 years) are found.
 After reconstruction secondary changes such as an increase in adipose and fibrous tissue may be treated using lipolymphosuction. Alternatively, localized resection may act as an adjunct to the bypass procedures.
After reconstruction secondary changes such as an increase in adipose and fibrous tissue may be treated using lipolymphosuction. Alternatively, localized resection may act as an adjunct to the bypass procedures.
 The ultimate goal of reconstruction using microsurgical grafting is to come as close as possible to the original status and to avoid additional ongoing treatment.
The ultimate goal of reconstruction using microsurgical grafting is to come as close as possible to the original status and to avoid additional ongoing treatment.
Introduction
Vascular surgery routinely deals with obstructions of the vascular system. These obstructions are dealt with using bypass techniques. With advances in microsurgical technique and instrumentation, the application of bypass techniques to the lymphatic system is now also possible.1 Furthermore, just as parts of the vascular system can be used as grafts, e.g., vein grafts, segments of these lymphatic channels can also be used as grafts.2,3
Historical perspective
Various surgical procedures have been used to treat lymphedema of the extremities with varying degrees of success.4–10 These operative strategies can be classified into two categories: ablative operations and physiological operations.
Ablative operations
The first reported surgical procedure for lymphedema was published in 1912 by Charles, who described a procedure for scrotal lymphedema and its application to lower limb lymphedema.5 The original operation involved the complete and circumferential resection of the skin, subcutaneous tissue, and deep fascia, followed by split-thickness skin grafting. However, this procedure was associated with significant complications and follow-up studies revealed hyperkeratosis, papillomatosis, and ulcerations in the grafted areas.11,12 Modifications of this technique involved utilization of the resected skin rather than other donor sites, and performing the surgery in two stages.13–20 This reduced the morbidity and the rate of complications and, as the operation is currently performed, all overlying skin and soft tissue above the deep fascia in the lymphedematous area is resected, and the defect is covered by a skin graft harvested from the resected specimen (Fig. 3.1). Even though the morbidity is significant, the Charles procedure remains a reasonable option in very extreme cases.
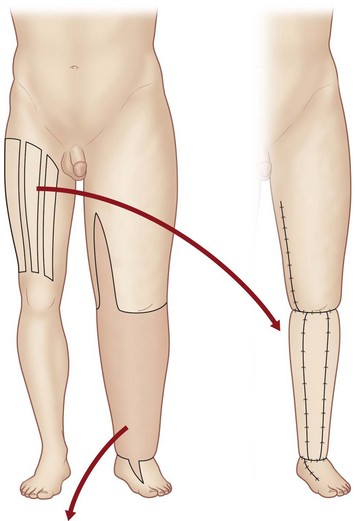
Fig. 3.1 The Charles operation.5 Skin and edematous soft tissue are excised, and split-thickness skin grafts are taken from the opposite thigh. The defect is covered by the skin grafts.
(Reproduced from Suami H, Chang DW. Overview of surgical treatments for breast cancer-related lymphedema. Plast Reconstr Surg. 2010;126:1853–1863.)
Resection of fascia and subcutaneous tissue and the creation of skin flaps was based on the proposals of Homans,6 Anchincloss, and Servelle.21–23 In contrast to the Charles procedure, the flap procedure yields a better cosmetic result, by retaining the overlying skin, but sufficient healthy skin in the affected extremities is necessary for this method to be feasible. The first surgical procedure described specifically to treat upper extremity lymphedema was probably the Sistrunk operation, reported in 1927.9,24 This procedure was a modification of the Kondoleon operation, in which excess skin and soft tissues are excised via a spindle-shaped incision in the medial side of the limb.7 Sistrunk added a more extensive removal of the deep fascia to the Kondoleon operation, with the goal of creating a spontaneous connection between the superficial and deep lymphatic vessels. These procedures combined ablation with an attempt at a physiologic solution to the treatment of lymphedema in an effort not only to reduce the bulk, but also to divert lymphatic flow.
Liposuction
A less invasive way to reduce the amount of subcutaneous tissue is through the use of liposuction. It was first described by Illouz as a method for treating lymphedema.25 In 1989, O’Brien and colleagues reported using liposuction for lymphedema.26,27 In 1998, Brorson and Svensson reported using the same technique and recommended liposuction as the preferred surgical procedure for treating lymphedema.28 Liposuction can be effective for initially reducing the volume of hypertrophic adipose tissue, but it has a risk of damaging the residual lymphatic vessels and thus exacerbating the lymphedema.29
Physiological operations
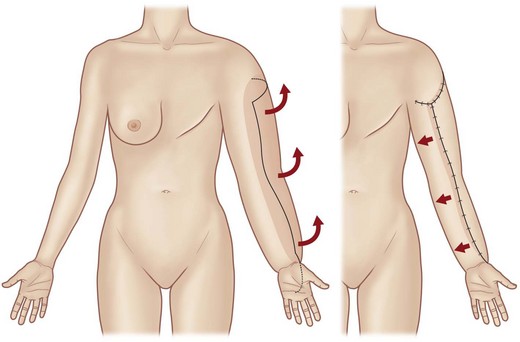
The first attempts to divert lymph from the subcutaneous to the muscular compartment through partial or complete resection of the fascia were described by Lanz and Kondoleon.34–36 Redirection of lymph from the superficial to the deep compartment is also a component of the Thompson method. Thompson refined surgery for upper extremity lymphedema by raising a de-epithelialized, rectangular “hinge” skin flap from the entire length of the upper limb and embedding the tip of the flap beside the deep neurovascular bundle (Fig. 3.2).37,38 This operation was also designed to create a lymphatic bridge between the superficial and deep lymphatic systems.38–44
Facilitating spontaneous lympholymphatic anastomoses within the subcutaneous tissue has been the aim of several methods and a variety of flaps were created for transposition into the edematous area.33,45–47 Both Sistrunk and Thompson attempted to create a new lymphatic drainage pathway by inducing the retained lymph fluid in the superficial lymphatic system to drain into the deep lymphatic system. However, there was no objective evidence that their surgical attempts to create these pathways succeeded.
Greater omentum flaps
Goldsmith proposed the creation of a pedicle from the greater omentum because the greater omentum contains many lymphatic vessels.30,31 In the procedure of Goldsmith et al., the greater omentum was raised from the abdomen with the ipsilateral gastroepiploic vessels preserved; the flap was then transferred to the lymphedematous upper limb through a subcutaneous tunnel in the chest. The lymph fluid from the upper limb was expected to drain into the abdomen through the lymphatic vessels along the vascular pedicle. However, there was no objective evidence that the transferred omentum was effective in transporting excess lymph fluid from the lymphedematous limb, and the operation was phased out because of its high risk of morbidity. Kinmonth et al. meanwhile devised the construction of an enteromesenteric bridge, in which a pedicle of ileum (denuded of its mucosa) and its mesentery (rich in lymphatics) are used to drain lymph out of the edematous tissue.48
Vein grafts
The use of veins for reconstruction of an interrupted lymphatic system was investigated by Holle and Mandl experimentally and performed in 2 patients clinically.49,50 Campisi et al. reported on a larger series with this technique.51
Currently the most popular way to drain lymph from edematous tissue is the construction of connections between the lymphatic and the venous system in the periphery. First reports on lymphonodular and lymphovenous anastomoses were provided by Allen and Taylor,52 Laine,53 Nielubowicz and Olszewski,54 and Rivero et al.55 Degni designed a special needle to facilitate the insertion of lymphatic vessels into veins.56,57
Further improvements were described by O’Brien et al.58–61 using microsurgical techniques. In some patients, excisional methods were combined with lymphovenous shunting. A large cohort of patients successfully treated with microsurgical lymphovenous anastomosis was reported by Campisi et al.62 However, not everyone can replicate these results and experimental studies showed problems with thrombotic occlusion at the site of anastomosis, with a patency of 20% after 5 months of follow-up.63–65 Specific preparations that ensured an undisturbed connection to the venous valve led to an improved patency rate of 44% after 6 months.66,67 An improved way to divert lymphatic fluid to the venous system was described by Becker et al.68–70 and Trevidic and Cormier.71 Lymph nodes included in a microvascular free flap theoretically promote spontaneous lympholymphatic anastomoses and divert the lymph via the lymph node to the venovenous anastomoses of the flap and thereby back to the venous system.
Reconstruction methods
Direct reconstruction of the lymphatic system became a conceivable possibility only after the advent of microsurgery. Prior to that it was commonly felt that it was impossible to anastomose lymphatic vessels because of their extremely small diameter. Danese et al.72 approximated lymphatic vessels as close to each other as possible and waited for spontaneous regeneration. They were able to demonstrate transport of contrast medium via the lymphatics with this technique. In a patient suffering from lymphedema of the arm, they mobilized two lymphatic channels proximally and distally, approximated them in the axilla, and achieved reduction of the edema.73
Subsequent approaches included interpositioning of veins49–51 between lymphatic vessels and implanting free flaps with lymphatic vessels.74,75 Campisi advocated using a vein interposition graft between the lymphatic vessel bundles above and below the site of lymphatic blockage to bypass the obstructed area (Fig. 3.3).76 In this procedure, multiple lymphatic vessels are inserted into the distal cut end of a vein graft and secured by sutures, and lymphatic vessels in the supraclavicular area are anastomosed to the other end of the vein graft.
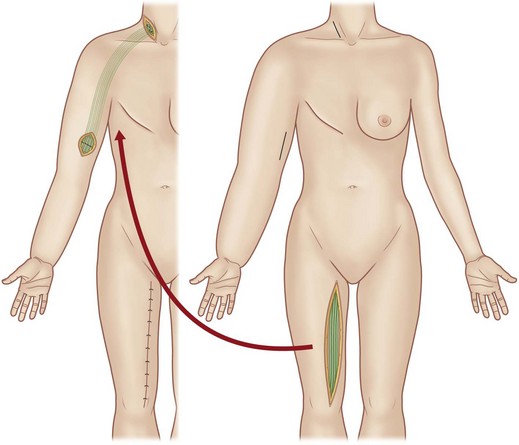
Fig. 3.3 The vein interposition graft procedure of Campisi.76 A vein graft is harvested from the great or small saphenous vein or the anterior forearm. The vein graft is inserted between the lymphatic vessels above and below the site of lymphatic blockage.
The first attempts to anastomose lymphatic vessels were made by Baumeister et al.,1 Acland and Smith,77 and Cordeiro and coworkers.78 The first successful therapeutic lympholymphatic grafting was performed in 1980 by Baumeister in a patient with unilateral lymphedema of the lower extremity,3,79,82 after extensive animal experiments on thoracic duct transplants in rats2 and treatment of experimental lymphedema in dogs, using lymphatic autografts.1
Baumeister and colleagues2,3 reported an approach to upper and lower extremity lymphedemas in which healthy lymphatic vessels from the medial thigh area are used as grafts. Ho and colleagues83 reported an approach for upper-extremity lymphedema using a composite graft including the greater saphenous vein. The ventromedial lymphatic bundle of the thigh consists of about 16 lymphatic channels.84 Therefore this region is useful for harvesting provided the knee and the inguinal region, where lymphatic channels are confluent, are avoided. Depending on the length of the thigh, lymphatic grafts can be harvested up to about 30 cm in length. The lymphatic graft is inset under the skin of the front of the shoulder to create lymphatic bypass routes between the upper arm and the supraclavicular region (Fig. 3.4). Lymphatic vessels at each end of the graft are identified under the microscope and anastomosed to recipient lymphatic vessels in the neck and upper arm in accordance with the flow direction of the donor lymphatic vessels.
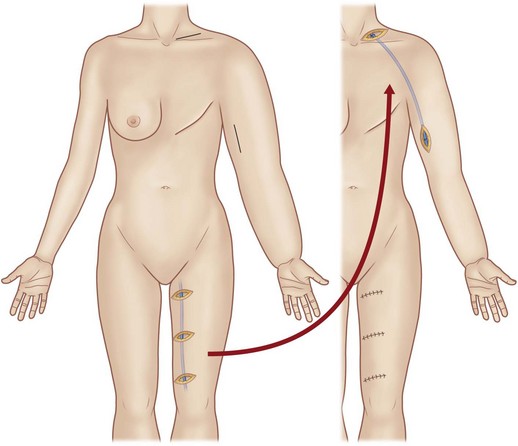
Fig. 3.4 The lymphatic graft operation of Baumeister and Siuda.79 A lymphatic graft is harvested from the medial aspect of the thigh. The graft is inset subcutaneously, and lymphatic vessels at each end of the graft are anastomosed to recipient lymphatic vessels to create a lymphatic bypass route between the upper arm and neck.
(Reproduced from Suami H, Chang DW. Overview of surgical treatments for breast cancer-related lymphedema. Plast Reconstr Surg. 2010;126:1853–1863.)
Baumeister and Siuda79 verified the postoperative patency of the lymphatic vessels within the graft using lymphoscintigraphy; postoperative images demonstrated newly created lymph pathways as well as faster clearance of radioisotope than shown in preoperative images.83,85 The resulting volume reduction in the affected upper limb was maintained in patients for 3 years after the operation. However, the lymphatic graft operation leaves a long scar at the donor site and also has a risk of precipitating the development of lymphedema in the donor leg where the lymphatic graft was harvested.
Lymphovenous bypass
The concept of a lymphovenous bypass operation was first described by Laine in 1963 in a rat model.53 To redirect excess lymph fluid from the lymphedematous limb into the venous system, peripheral lymphatic vessels were anastomosed to small veins using microsurgical techniques. Later in the 1960s, Yamada performed similar experiments in dogs and then applied the technique to treat lower limb lymphedema in human patients.67 In the 1970s, O’Brien and colleagues reported using the same approach to treat lymphedema; since then, many others have refined the technique.59,67,86,87
Various lymphovenous anastomosis techniques have been described. Sedlacek described a technique of end-to-side lymphovenous anastomosis to implant the end of a lymphatic vessel inside the lumen of the saphenous vein.88 Degni modified this method by using a double-ended microneedle to anchor a lymphatic vessel to the venous wall and a grooved needle for a suture guide.56,89 Yamada described a technique of end-to-end anastomosis between a lymphatic vessel and a venule, often referred to as lymphaticovenular bypass.67 More recently, Yamamoto and Sugihara described technique where a group of severed lymphatic vessels are implanted into a large vein.10 Most recently, using supermicrosurgical techniques, Koshima et al. used lymphaticovenular anastomosis as a treatment for lymphedema.90
Basic science/disease process
Lymphedema is characterized by an imbalance between the lymphatic load and the lymphatic transport capacity. The lymphatic load represents the amount of lymphoid fluid that has to be transported via the lymphatic system within a given time frame within a specific part of the body, e.g., an extremity. The lymphatic transport capacity on the other hand is the amount of lymphatic fluid that can be maximally transported by the lymphatic system. It is dependent on the number and functional status of the lymphatic vessels and the lymph nodes.91 When the lymphatic load outstrips the transport capacity lymphedema results. This imbalance can have a number of causes, as already described, and may be congenital or acquired. As lymphedema progresses some changes occur in the tissues such that there is an increased amount of adipose tissue as well as fibrous tissue. Furthermore, the skin becomes thickened, firm, and unyielding and ultimately takes on an appearance similar to what one would imagine as elephant skin, hence the term “elephantiasis.”
Classification and etiology of lymphedema
Lymphedema is classified as primary or secondary. Two types of primary lymphedema are recognized:
1. Type I (Nonne–Milroy): the familial congenital type is based on a vascular endothelial growth factor-receptor-3 mutation
2. Type II (Meige): can be seen as lymphedema praecox, arising during adolescence, and as lymphedema tarda, with an onset after the age of 35.
Secondary lymphedema is due to acquired damage to the lymphatic system.
Diagnosis/patient presentation
The clinical picture is characterized by increased tissue thickness and decreased tissue pliability.
Direct-contrast lymphography, using oily contrast medium and invasive administration via transected lymphatic vessels, was introduced by Kinmonth and greatly advanced our knowledge of the lymphatic system.92 However, due to the invasiveness of the application (and injury to the lymphatic vessels and lymph nodes), it was found to cause worsening of lymphedema.
Indirect-contrast lymphography, using water-soluble contrast medium that is injected subepidermally, is unable to visualize lymphatic vessels to an extent comparable with direct lymphography and gained only limited use.93 In primary lymphedema this technique might be used to evaluate whether there are any lymphatic vessels present in the periphery and if so, if they might be able to transport lymph towards a proximally performed anastomosis.
First attempts to visualize lymphatic vessels with magnetic resonance imaging (MRI) using contrast medium administered subdermally are promising. This will prove useful for exact planning prior to reconstructive procedures as well as examining the patency of lymphatic grafts without damaging them.94 For the detection of vascular lymphatic malformations, MRI is extremely valuable both with and without the use of contrast medium.
For routine procedures, the key diagnostic tool, aside from the clinical evaluation, is lymphoscintigraphy. It can be repeated and used for diagnostic as well as for follow-up purposes. It gives quite a good impression of the function and visualizes routes of lymphatic flow. Introducing the lymphatic transport index (TI), which summarizes the findings derived from the lymphoscintigraphic studies, allows for a semiquantitative evaluation of the lymphatic flow without the need for standardized physical movement on the part of the patient. It ranges from TI = 0 for an optimal lymphatic outflow to TI = 45 for no visible flow. Normal values are below 10. This transport index also provides a good basis for follow-up studies and can show lymphatic flow along the route of lymphatic grafts.85,95
Different approaches are described to improve exact quantification of lymphatic flow. When measuring regions of interest, it is critical to standardize the application of the radiopharmaceutical and the physical movements performed by the patient during the procedure.96 The visualization of cutaneous lymphatic vessels using duplex sonography is controversial and is of limited value for surgeons. Another diagnostic tool that can be used is the subepidermal injection of Patent Blue dye. Normally, lymphatic transport is visualized in the superficial lymphatic collecting system. In pathologic situations, the so-called dermal back-flow leads to pooling of the contrast within the skin that results in a “cloud”-like appearance. Since allergic reactions have been reported, staining of lymphatic vessels with Patent Blue dye is generally performed during surgery under general anesthesia.