4 Lower extremity sarcoma reconstruction
Synopsis
 Any lesion in the lower extremity with a clinical history of pain, continuous growth, size over 5 cm or deep subfascial localization is suspicious of a sarcomatous malignancy and should be surgically biopsied according to established surgical rules.
Any lesion in the lower extremity with a clinical history of pain, continuous growth, size over 5 cm or deep subfascial localization is suspicious of a sarcomatous malignancy and should be surgically biopsied according to established surgical rules.
 The single statistically proven modality of curing sarcomas and prolonging postsurgical lifespan remains surgical excision with wide margins, resulting in a postoperative R0 status. To date, no other neoadjuvant or postoperative treatment modality can replace this approach. If wide margins cannot be achieved, adjuvant therapy is indicated for extremity preservation.
The single statistically proven modality of curing sarcomas and prolonging postsurgical lifespan remains surgical excision with wide margins, resulting in a postoperative R0 status. To date, no other neoadjuvant or postoperative treatment modality can replace this approach. If wide margins cannot be achieved, adjuvant therapy is indicated for extremity preservation.
 Plastic surgical lower extremity sarcoma reconstruction, especially in bone sarcoma reconstruction, is the classical field of an interdisciplinary, multimodal approach, most commonly together with tumor and orthopedic surgeons, oncologic radiotherapists, and oncologists.
Plastic surgical lower extremity sarcoma reconstruction, especially in bone sarcoma reconstruction, is the classical field of an interdisciplinary, multimodal approach, most commonly together with tumor and orthopedic surgeons, oncologic radiotherapists, and oncologists.
 Modern oncoplastic reconstructive surgery can provide adequate reconstructive options for almost any defect size and composition, so radical tumor excision can be combined with over 95% extremity preservation today.
Modern oncoplastic reconstructive surgery can provide adequate reconstructive options for almost any defect size and composition, so radical tumor excision can be combined with over 95% extremity preservation today.
 The plastic reconstructions in sarcoma-related limb-sparing surgery are often demanding and complex and consist of the full spectrum of plastic surgical options. They should be performed in specialized centers and specifically adapted to the patient and case profile.
The plastic reconstructions in sarcoma-related limb-sparing surgery are often demanding and complex and consist of the full spectrum of plastic surgical options. They should be performed in specialized centers and specifically adapted to the patient and case profile.
Introduction
Sarcomas in the lower extremity
Sarcomas in the lower extremity are more common than in the upper limb (74 versus 26%) and represent the most common location of sarcomas in the body overall (45%).1 Currently, they can be safely treated by extremity preservation in most cases, if properly performed according to the rules described in this chapter and in the pertinent current literature. In this context, several studies have now demonstrated that limb-sparing surgery is oncologically not inferior to amputation in the treatment of lower extremity sarcoma (see Outcomes, prognosis, and complications, below). While amputation was the keystone of previous surgical therapy several decades ago, it usually represents only an important last-line therapeutic modality today. The common misconceptions that amputations have a better outcome in both tumor safety and quality of life have both definitely been proven wrong.2–4
Due to the highly functional anatomy of our extremities with vessels, nerves, tendons, bones, and muscles in close vicinity, even smaller tumors can represent a challenge to both the resecting tumor surgeon as well as the reconstructive plastic surgeon. Preservation of the lower extremity in sarcoma reconstruction differs from similar manifestations in the upper extremity5 in several key points:
• Stability and weight-bearing capability are usually held in higher regard than functional mobility or range of motion in the lower extremity.
• Postoperative appearance is usually less important. In most urban cultures the reconstructed legs with their scars and possible voluminous flaps can easily be hidden in clothing and have a less important role in social interaction than the upper extremity (i.e., shaking hands).
• Weight-bearing demands are higher and atherosclerotic vessel damage and orthostatic venous pressure are more profound in the lower extremity: Both may play a major role in free tissue transfer.
• Nerve regeneration is less successful in the lower extremity at any age.
• Wound healing is slower and the risk of infectious complication is higher.
Basic science/disease process
Epidemiology soft-tissue sarcomas
Soft-tissue sarcomas are a rare disease entity with an incidence of 1 : 100 000 in adults and 10–15% in children. This accounts for an incidence of about 10 600 new cases in 2009 in the US, representing 1–2% of all malignancies (www.seer.cancer.gov). There is no overall significant gender predisposition and the overall median age at presentation is 50–60 years.
With about 45% of all sarcomas occurring in the lower extremity, 15% in the upper extremity, 10% in the head and neck region, 15% in the retroperitoneal space, and the remaining 15% in the abdomen and the chest wall,6 the musculoskeletal system of the extremities and the abdominal and thoracic walls is the most common predilection site. Extremity sarcomas are most common in the thigh (50–60%).
While most cases of soft-tissue sarcomas are sporadic, there are some genetic and nongenetic risk factors, summarized in Table 4.1. Up to 60% of all soft-tissue sarcomas contain a somatic mutation of p53.7 A detailed description of the various risk factors is beyond the scope of this chapter, but there are several strong associations to be mentioned: a history of radiation exposure accounts for up to 5.5% of all sarcomas. The risk is dose-dependent and the latency period between radiation and clinical tumor manifestation is around 5 years. Over 80% of radiation-associated sarcomas are high-grade types.8 Neurofibromatosis type NF-1 is strongly associated with the cumulative lifetime risk of up to 13% for the occurrence of malignant peripheral nerve sheath tumors.
Table 4.1 Predisposing factors for soft-tissue sarcomas
| Genetic | Neurofibromatosis NF-1 (von Recklinghausen disease) |
| Retinoblastoma | |
| Gardner’s syndrome | |
| Werner’s syndrome | |
| Bloom’s syndrome | |
| Fumarate hydratase leiomyosarcoma syndrome | |
| Diamond–Blackfan anemia | |
| Mechanical | Li–Fraumeni syndrome |
| Postparturition | |
| Chemical | Chronic irritation |
| Polyvinylchloride | |
| Hemochromatosis | |
| Dioxin (TCDD): “Agent Orange” | |
| Radiation | Arsenic |
| Traumatic/accidental | |
| Lymphedema | Posttherapeutic |
| Parasitic (filariasis) | |
| Iatrogenic | |
| Stewart–Treves syndrome | |
| Infectious (viral) | Congenital |
| Kaposi sarcoma (human herpesvirus-8) |
The sarcoma subtype is determined by light and electron microscopy, immunohistochemistry, and cytogenetic analysis. If it results in a tumor that cannot be designated accordingly, a descriptive evaluation is given for an “unclassified sarcoma.” Obtaining reference pathologies for soft- and bone tissue sarcomas should be standard, as the rate of diagnostic agreement among specialists is below 75%.9,10
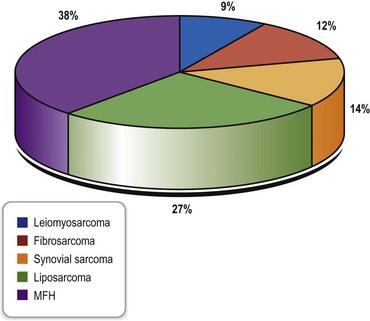
The most common histopathologic subtype distribution in extremities in the largest series in the literature is shown in Figure 4.1.
Bone sarcomas
About 2600 new primary bone sarcomas occur each year in the US (www.seer.cancer.gov). The overall median age at diagnosis is 39 years. Many predisposing factors for bone sarcomas are similar to those for soft-tissue sarcomas (retinoblastoma, Li–Fraumeni syndrome, radiation, and others) (Table 4.1). Paget’s disease, bone infarction, and fibrous dysplasia may also represent risk factors for bone sarcomas.
The most common type is the osteogenic sarcoma, which has a predilection for the metaphyses around the knee in about 50% of cases. It is the third most common cancer in the young (www.nhs.uk) with a second peak around age 60. The male-to-female ratio is almost 2 : 1 in large studies and for this specific tumor the median age at diagnosis is 17 years. Only 6.4% present initially with pathological fractures, whereas the majority are detected in the workup of a painful mass or swollen extremity.11,12 It commonly arises in the medulla, but as a juxtacortical osteogenic sarcoma it arises from the external surface, most commonly the posterior aspect of the femur.
Tumor growth and metastasizing
Hematogenic spread is most common in soft-tissue and bone sarcomas. For lower extremity tumors, the primary site for metastasis is the lung. Lymphatic metastases are present in less than 5% of all soft-tissue sarcomas (rhabdomyosarcoma, angiosarcoma, epithelioid-like sarcoma).13,14
Historical perspective
However, the mortality rate of radiated patients equaled the mortality rate of patients who underwent amputation. A first neoadjuvant protocol was developed in 1940 by Cade and Ferguson,15 who used preoperative radiation followed by amputation 6 months later in metastasis-free patients. The aim of the protocol was to avoid unnecessary amputation.
Episodic anecdotes reporting limb salvage appear as early as 1895, when Mikulicz described two resection arthrodeses of the knee for distal femoral lesions in Europe.16 Sauerbruch in Germany described his “Umkippplastik” in 1922 as the precursor of today’s rotationplasty.17 A first systematic approach to limb salvage was suggested in 1940 by Phemister in his article “Conservative surgery in the treatment of bone tumors”.18,19
Diagnosis/patient presentation/imaging
A detailed history and physical examination are the first initial and important steps to professional tumor surgery. In sarcomatous lesions, the patient often relates the tumor causally to an – often minor – traumatic event that brings the lesion to the patient’s attention. Acute trauma, however, is not a proven predisposing factor for sarcoma development. Because of this, there is often a considerable time lag between this initial recognition and the first presentation of the lesion to a medical professional. Furthermore, the rationale of the lesion is often erratically misinterpreted and then causes a variety of inadequate treatments by both lays and physicians, further delaying proper diagnosis. The average duration of any symptoms before seeing a physician is 6 months in all soft-tissue sarcomas, but possibly shorter in extremity manifestation.3 So in adults, lesions that: (1) have not disappeared after 4 weeks; (2) are located subfascially or in the popliteal or groin flexion creases; (3) continue to grow or are symptomatic (i.e., pain, paresthesias); or (4) are already larger than 5 cm on detection should generally be biopsied as they are highly suspicious for malignancy.
Modern spiral CT scans are indispensable for clarifying the detailed anatomy of osseous sarcomas, determining the effect on skeletal structures of neighboring soft-tissue tumors, and aiding in operative planning of these sarcomatous entities. Thoracic and abdominal CT scans are the diagnostics of choice for staging of high-grade sarcomas of the extremities and detect intrapulmonary and abdominal metastases. In recurrent disease, positron emission tomography (PET) CT can augment information about suspicious lesions in selected cases, though it is not accepted as a standard instrument for preoperative workup.20–22
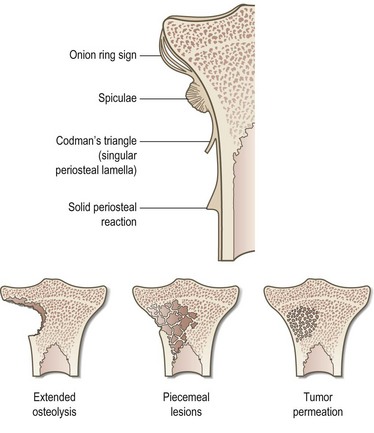
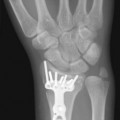
Plain X-ray films demonstrate specific periosteal or cortical signs, osteolyses and paraosseous calcifications in diaphyseal and metaphyseal bony lesions. Even today, a plain radiograph remains the diagnostic method of choice for primary bone sarcomas (Fig. 4.2). A plain chest radiograph is still considered the standard for clinical staging in low-grade extremity lesions.
A special laboratory workup for soft-tissue sarcomas does not exist, whereas elevated alkaline phosphatase and lactate dehydrogenase over 400 U/L are independent predictors of an unfavorable outcome in bone sarcomas.23
Patient profile/general considerations/treatment planning
Treatment planning
Finally, tumor staging is done according to the current staging systems for soft-tissue sarcomas. The American Joint Committee on Cancer (AJCC) system is designed for extremity sarcomas (Table 4.2) including most, but not all, histologic subtypes. Dermatofibrosarcoma protuberans and angiosarcoma, among others, are exempt from AJCC staging. For primary bone sarcomas like osteogenic sarcoma, the Muskuloskeletal Tumor Society staging system is used (Table 4.3).
Table 4.2 American Joint Committee on Cancer staging system for soft-tissue sarcomas
| Classification and staging | Characteristic |
|---|---|
| Primary tumor (T) | |
| T1 | Tumor 5 cm or less in greatest dimension |
| T1a | Superficial tumor (in relation to investing fascia) |
| T1b | Deep tumor (visceral and retroperitoneal sarcomas are defined as deep tumors) |
| T2 | Tumor larger than 5 cm in greatest dimension |
| T2a | Superficial tumor |
| T2b | Deep tumor |
| Regional lymph nodes (N) | |
| N0 | No evidence of nodal metastasis |
| N1 | Nodal metastasis present |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| Grade (G) | |
| G1 | Low-grade |
| G2 and G3 | High-grade |
| Staging | |
| Stage I | Low-grade tumors, no evidence of regional nodes or distant metastases (T1a, T1b, T2a, T2b) |
| Stage II | High-grade, small tumors (T1a and b), and superficial, large tumors (T2a), no evidence of regional nodes or distal metastases |
| Stage III | High-grade, deep tumors larger than 5 cm (T2b), no evidence of regional nodes or distant metastases |
| Stage IV | Any tumor with regional nodes or distant metastases |
(Reproduced from Papagelopoulos PJ, Mavrogenis AF, Mastorakos DP, et al. Current concepts for management of soft tissue sarcomas of the extremities. J Surg Orthop Adv 2008;17:204–215.)
Table 4.3 Muskuloskeletal Tumor Society staging system
| Stage | Characteristic |
|---|---|
| IA | Low-grade, intracompartmental |
| IB | Low-grade, extracompartmental |
| IIA | High-grade, intracompartmental |
| IIB | High-grade, extracompartmental |
| IIIA | Low- or high-grade, intracompartental with metatases |
| IIIB | Low- or high-grade, extracompartental with metatases |
(Reproduced from Papagelopoulos PJ, Mavrogenis AF, Mastorakos DP, et al. Current concepts for management of soft tissue sarcomas of the extremities. J Surg Orthop Adv 2008;17:204–215.)
Radiotherapy
Radiotherapy is the primary adjunctive treatment method in sarcoma management today. Neoadjuvant radiation in large sarcomas uses external-beam irradiation, which helps in tumor shrinkage, thickening of the tumor capsule that facilitates adequate resection and the achievement of negative margins during wide resection, and reduces potential surgical tumor seeding. The disadvantages of preoperative irradiation are a higher rate of wound-healing complications compared to postoperative radiotherapy and the creation of necrotic tumor material for the pathologist.2
Treatment/surgical resection techniques
Biopsy techniques
Fine-needle or core needle aspirations
These techniques gain only a very small amount of tissue, even in the hands of an experienced clinician. Although tissue aspiration with a 23-gauge needle usually harvests only a very small number of cells, the volume of tissue gained from a core-needle biopsy is slightly higher. Still, both methods have the disadvantage of not representing the tumor tissue components correctly, especially in larger tumors, which makes it very difficult for the histopathologist to find the exact diagnosis, perform the necessary number of different studies, and determine the correct grading. However, if combined with CT scan or ultrasound-guided needle placement, core biopsies may achieve a correct diagnosis in up to 90% of cases under optimal circumstances. Fine-needle aspirations only reach 56–72%.24–26 Although single core biopsies may harvest too little tissue for an extensive pathological workup with several stainings and immunohistochemical diagnostics, they have the advantage of gathering tissue from different parts of the tumor to create a comprehensive picture.26
Both methods are atraumatic and only very rarely cause dangerous tumor cell-dissipating hematomas. In several institutions, core biopsies are reserved for surgically unresectable tumors (i.e., retroperitoneal lesions) to determine tissue type and guide an eventual neoadjuvant therapy,3 while others use it as a prime diagnostic tissue-sampling method.26
In summary, fine-needle biopsies do not have a place in sarcoma diagnosis in lower extremities, and both core biopsy and open surgical biopsy are very operator-dependent: If poorly performed, the risk of getting an inadequate diagnostic sample or tumor seeding is high.27,28
Bone-forming lesions are very difficult to sample adequately percutaneously and open biopsies are preferable.29
Excisional biopsy
Meticulous hemostasis and the placement of a closed suction drainage within the wound to prevent possible tumor seeding through a hematoma are paramount before a layered skin closure is done. The skin should be closed with single interrupted stitches or intracutaneous sutures. Mattress sutures or separate drainage perforations leave stitch marks too far away from the incision: as they all have to be excised in case of malignancy, this would enlarge the amount of tissue to be resected. A sterile circular compressive dressing is placed and the affected extremity is immobilized, especially in procedures close to joints. Temporary splinting for a few days assists well here.3